The Performance of Real-Time Polymerase Chain Reaction in Patients with Scanty Positive Acid-Fast Bacilli Sputum Smear in Diagnosis of Pulmonary Tuberculosis: 5-Year Retrospective Study
DOI:
https://doi.org/10.33192/Smj.2021.58Keywords:
Performance, scanty acid fast bacilli, pulmonary tuberculosis, polymerase chain reactionAbstract
Objective: To assess the performance of real-time polymerase chain reaction (RT-PCR) to diagnosis pulmonary tuberculosis in patients with scanty positive acid-fast bacilli sputum smears, in a single hospital.
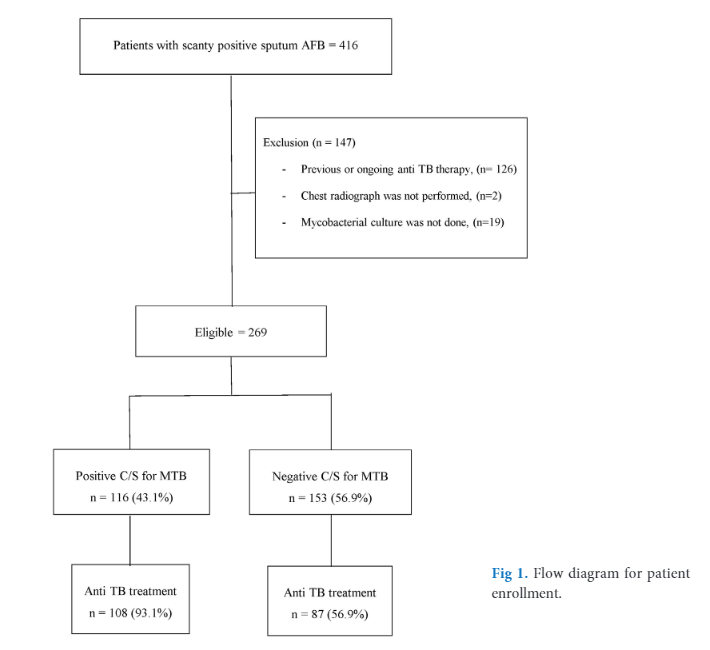
Materials and Methods: All patients, who had scanty positive AFB sputum smears in Songklanagarind Hospital; between 2015 and 2019 were included. Demographic data, clinical data, radiographic findings, RT-PCR and mycobacterial culture results were reviewed.
Results: From a total of 269 patients reporting scanty AFB smears, 116 patients (43.1%) had cultures confirmed as M. tuberculosis. From overall, samples from 92 patients with scanty AFB smear were processed for RT-PCR. There were 26 (28.3%) isolates having positive RT-PCR test results. Of these 26 isolates that RT-PCR positive, 25 (96.2%) were culture positive, while only 1 (3.8%) were culture negative. A remaining 66 samples that RT-PCR negative, 15 (22.7%) were culture positive for tuberculosis. Using mycobacterial cultures as the gold standard, the sensitivity, specificity, positive predictive value and negative predictive value of RT-PCR were 62.5%, 98.1%, 96.2%, and 77.3%, respectively. Pulmonary consolidation and cavity on chest radiograph were associated with the growth of M. tuberculosis, with an OR of 2.3 (95% C.I. 0.26-0.73) and 3.4 (95% C.I. 1.2-9.9), respectively.
Conclusion: Less than half of the patients with scanty smears had culture-confirmed tuberculosis; RT-PCR also has low sensitivity. Consequently, a negative RT-PCR does not exclude tuberculosis; especially in cases of a high index for clinical suspicion. Radiographic findings; including pulmonary consolidation and cavities, are helpful predictors for supporting this diagnosis.
References
2. World Health Organization. Regional Office for South-East Asia. Tuberculosis control in the South-East Asia Region: Annual Report 2016. Available from: https://apps.who.int/iris/handle/10665/205286 [cited 2020 Dec 9]
3. Glaziou P, Falzon D, Floyd K, Raviglione M. Global epidemiology of tuberculosis. Semin Respir Crit Care Med 2013;34(1):3-16.
4. Cattamanchi A, Davis JL, Worodria W, Boon S, Yoo S, Matovu J, et al. Sensitivity and Specificity of Fluorescence Microscopy for Diagnosing Pulmonary Tuberculosis in a High HIV Prevalence Setting. Int J Tuberc Lung Dis 2009;13(9):1130-6.
5. Young DB, Perkins MD, Duncan K, Barry CE. Confronting the scientific obstacles to global control of tuberculosis. J Clin Invest 2008;118(4):1255-65.
6. Monkongdee P, McCarthy KD, Cain KP, Tasaneeyapan T, Nguyen HD, Nguyen TNL, et al. Yield of Acid-fast Smear and Mycobacterial Culture for Tuberculosis Diagnosis in People with Human Immunodeficiency Virus. Am J Respir Crit Care Med 2009;180(9):903-8.
7. Githui W, Kitui F, Juma ES, Obwana DO, Mwai J, Kwamanga D. A comparative study on the reliability of fluorescence microscopy and Ziehl-Neelsen method in the diagnosis of pulmonary tuberculosis. East Afr Med J 1993;70(5):263-6.
8. Prasanthi K, Kumari AR. Efficacy of fluorochrome stain in the diagnosis of pulmonary tuberculosis co-infected with HIV. Indian J Med Microbiol 2005; 23(3):179–85.
9. Kivihya-Ndugga LE, van Claaff MR, Githui WA, Nganga LW, Kibuga DK, Odhiambo JA, et al. A comprehensive comparison of Ziehl-Neelsen and fluorescence microscopy for the diagnosis of tuberculosis in a resource poor urban setting. Int J Tuberc Lung Dis 2003;7(12):1163-71.
10. Singh NP, Parija SC. The value of fluorescence microscopy of auramine stained sputum smears for the diagnosis of pulmonary tuberculosis. Southeast Asian J Trop Med Public Health 1998;29(4):860-3.
11. Hanscheid T, Ribeiro CM, Shapiro HM, Perlmutter NG. Fluorescence microscopy for tuberculosis diagnosis. Lancet Infect Dis 2007;7(4):236-7.
12. Steingart KR, Henry M, Ng V, Hopewell PC, Ramsay A, Cunningham J, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis 2006;6(9):570-81.
13. Enarson DA, Rieder HL, Arnadottir T, Trébucq A. Management of tuberculosis: a guide for low income countries. 5th ed. Paris, France: International Union Against Tuberculosis and Lung Disease; 2000.
14. World Health Organization. Laboratory services in tuberculosis control. WHO/TB/98.258. Geneva: WHO; 1998.
15. World Health Organization. Definitions and reporting framework for tuberculosis–2013 revision. World Health Organization; 2013.
16. Lee JS, Kim EC, Joo SI, Lee SM, Yoo CG, Kim YW, et al. The incidence and clinical implication of sputum with positive acid-fast bacilli smear but negative in mycobacterial culture in a tertiary referral hospital in South Korea. J Korean Med Sci 2008;23(5):767-71.
17. Van Deun A, Salim AH, Cooreman E, Daru P, Das AP, Aung KJ, et al. Scanty AFB smears: what’s in name. Int J Tuberc Lung Dis 2004;8(7):816-23.
18. Wei Z, Zhang X, Wei C, Yao L, Li Y, Zhang X, et al. Diagnostic accuracy of in-house real-time PCR assay for Mycobacterium tuberculosis: a systematic review and meta-analysis. BMC Infect Dis 2019;19(1):701.
19. Shinu P, Nair A, Singh V, Kumar S, Bareja R. Evaluation of rapid techniques for the detection of mycobacteria in sputum with scanty bacilli or clinically evident, smear negative cases of pulmonary and extra-pulmonary tuberculosis. Memórias do Instituto Oswaldo Cruz 2011;106(5):620-4.
20. Chumpa N, Kawkitinarong K, Rotcheewaphan S, Sawatpanich A, Petsong S, Tumwasorn S, et al. Evaluation of Anyplex™ II MTB/MDR kit’s performance to rapidly detect isoniazid and rifampicin resistant Mycobacterium tuberculosis from various clinical specimens. Mol Biol Rep 2020;47(4):2501-8.
21. Perry MD, White PL, Ruddy M. Potential for use of the Seegene Anyplex MTB/NTM real-time detection assay in a regional reference laboratory. J Clin Microbiol 2014;52(5):1708-10.
22. Igarashi Y, Chikamatsu K, Aono A, Yi L, Yamada H, Takaki A, et al. Laboratory evaluation of the Anyplex™ II MTB/MDR and MTB/XDR tests based on multiplex real-time PCR and melting-temperature analysis to identify Mycobacterium tuberculosis and drug resistance. Diagn Microbiol Infect Dis 2017;89(4):276-81.
23. Kubica G P. Correlation of acid fast staining methods with culture results for mycobacteria. Bull Int Union Tuberc 1980;55:117-24.
24. Mokhtari Z, Larbaoui D. Correlation of results of slightly-positive direct diagnosis with those obtained from culture. Bull Union Int Tuberc 1973;48:88-9.
25. Odubanjo MO, Dada-Adegbola HO. The microbiological diagnosis of tuberculosis in a resource-limited setting: is acid-fast bacilli microscopy alone sufficient? Ann Ib Postgrad Med 2011;9(1):24-9.
26. Lawson L, Yassin MA, Ramsay A, Emenyonu NE, Squire SB, Cuevas LE, et al. Comparison of scanty AFB smears against culture in an area with high HIV prevalence. Int J Tuberc Lung Dis 2005;9(8):933-5.
Published
How to Cite
Issue
Section
License
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














