Serum Theophylline Concentrations in very Preterm Neonates Receiving Intravenous Aminophylline for Apnea
DOI:
https://doi.org/10.33192/Smj.2021.68Keywords:
Aminophylline, apnea of prematurity, therapeutic drug monitoring, serum theophylline concentration, therapeutic drug levelAbstract
Objective: To determine the percentage of neonates who achieved therapeutic theophylline level (TTL) after receiving standard IV loading/maintenance aminophylline doses. To assess factors associated with achieving therapeutic theophylline concentrations and to describe adverse effects of aminophylline.
Materials and Methods: This was a pilot, cross-sectional study. Preterm neonates ≤34 weeks’ gestation for which aminophylline was indicated for treatment of apnea were enrolled. Standard IV aminophylline dosage is 8 mg/kg loading dose, followed by 1.5 mg/kg maintenance dose every 8 hours. Serum theophylline concentrations were measured prior to the 8th maintenance dose. Descriptive statistics, univariate and multivariate analyses were performed.
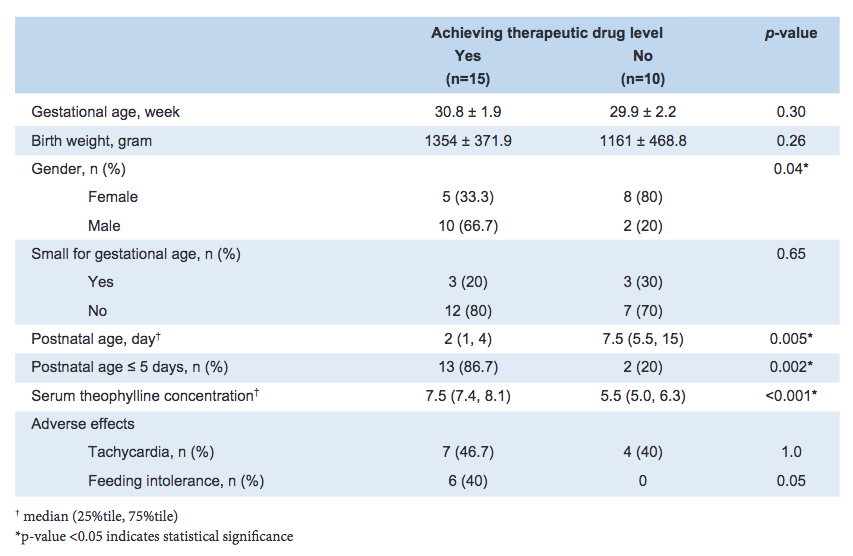
Results: Twenty-five neonates (52% female) were enrolled: mean (standard deviation) gestational age and birth weight were 30.4 (2) weeks and 1,277 (415) grams, respectively. Aminophylline was initiated at a median (25%tile, 75%tile) postnatal age of 4 (1, 8) days. Baseline heart rate prior to the loading dose was 153 (13) beats-per-minute. Sixty percent of neonates achieved a therapeutic theophylline level. In the univariate analysis, being male and postnatal age ≤5 days were associated with successfully achieving a TTL. After adjusting for gender, postnatal age ≤5 days was the only factor associated with achieving a TTL (adjusted odds ratio 17.7, 95% confidence interval: 1.9, 164.4). Tachycardia and feeding intolerance were observed in 44% and 24% of neonates, respectively.
Conclusion: Current IV aminophylline dosing conditions in Thailand achieved TTL in approximately two-thirds of neonates, suggesting therapeutic drug monitoring is beneficial for guiding dosing. A higher maintenance dose could be considered for neonates older than 5 days.
References
2. Martin RJ, Wang K, Koroglu O, Di Fiore J, Kc P. Intermittent hypoxic episodes in preterm infants: do they matter? Neonatology 2011;100:303-10.
3. Pillekamp F, Hermann C, Keller T, von Gontard A, Kribs A, Roth B. Factors influencing apnea and bradycardia of prematurity - implications for neurodevelopment. Neonatology 2007;91:155-61.
4. Kesavan K, Parga J. Apnea of prematurity: current practices and future directions. NeoReviews 2017;18:e149-e58.
5. Gal P, Roop C, Robinson H, Erkan NV. Theophylline-induced seizures in accidentally overdosed neonates. Pediatrics 1980;65:547-9.
6. Hochwald C, Kennedy K, Chang J, Moya F. A randomized, controlled, double-blind trial comparing two loading doses of aminophylline. J Perinatol 2002;22:275-8.
7. Bhatt-Mehta V, Schumacher RE. Treatment of apnea of prematurity. Pediatr Drugs 2003;5:195-210.
8. McClary JD. Therapeutic agents. In: Martin RJ, Fanaroff AA, Walsh MC, editors. Fanaroff & Martin's Neonatal-Perinatal Medicine Diseases of the Fetus and Infant. 11th ed. Philadelphia: Elsevier; 2020. p. 2018-27.
9. Bruel A, Roze JC, Flamant C, Simeoni U, Roussey-Kesler G, Allain-Launay E. Critical serum creatinine values in very preterm newborns. PLoS One 2013;8:e84892.
10. Choudhary M, Sharma D, Dabi D, Lamba M, Pandita A, Shastri S. Hepatic dysfunction in asphyxiated neonates: prospective case-controlled study. Clin Med Insights Pediatr 2015;9:1-6.
11. Jenjarat K, Chitsrisakda N, Suksumek N, Chamnanvanakij S. A randomized controlled trial comparing serum theophylline levels and side effects between two regimens of aminophylline in preterm infants. J Med Assoc Thai 2018;101:283-8.
12. Bhatt-Mehta V, Donn SM, Schork MA, Reed S, Johnson CE. Prospective evaluation of two dosing equations for theophylline in premature infants. Pharmacotherapy 1996;16:769-76.
Published
How to Cite
Issue
Section
License
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














