Cost-effectiveness Analysis Comparing Vonoprazanbased Triple Therapy with Proton Pump Inhibitorbased Therapy in the Treatment of Helicobacter pylori Infection in Thailand
DOI:
https://doi.org/10.33192/Smj.2021.107Keywords:
Cost-effectiveness, Vonoprazen, Proton pump inhibitors, Levofloxacin, Concomitant, Helicobacter pylori infectionAbstract
Objective: Helicobacter pylori (H. pylori) infection is one of the leading causes of gastrointestinal diseases such as dyspepsia, peptic ulcers. Thailand has a 45.9% prevalence of the infection and an increasing rate of resistance to clarithromycin, leading to standard treatments being less successful. Vonoprazan represents a novel drug offering a new treatment regimen. Although vonoprazan has been available in Thailand since 2019, its cost-effectiveness has not been studied previously.
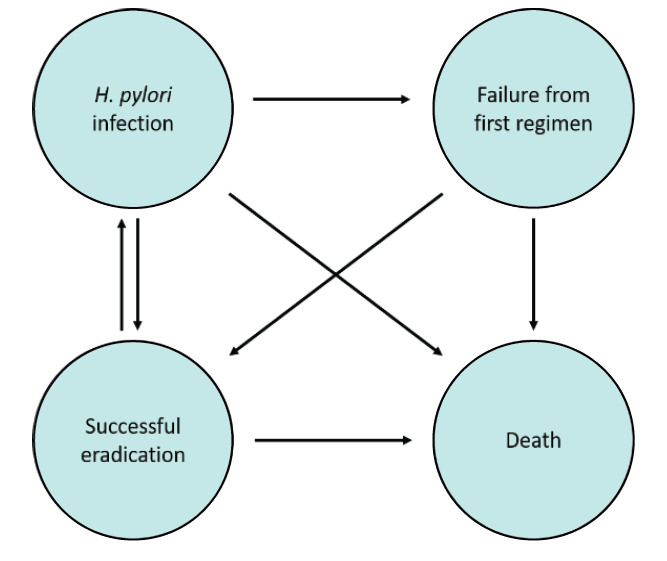
Materials and Methods: This study analysed the cost-effectiveness of vonoprazan-based triple therapy compared with PPI-based therapy, in treating clarithromycin resistant H. pylori, by using the markov model from a societal perspective.
Results: The total cost of vonoprazan-based triple therapy, levofloxacin-PPI based triple therapy and concomitant-PPI therapy were 784,932.08 baht, 783,863.65 baht and 783,874.55 baht respectively. The quality-adjusted life years (QALYs) of vonoprazan-based triple therapy, levofloxacin-PPI based triple therapy and concomitant-PPI therapy were 25.1118 years, 25.1147 years and 25.1054 years respectively. The cost-effectiveness ratio (CER) of vonoprazanbased triple therapy, levofloxacin-PPI based triple therapy and concomitant-PPI therapy were 31,257.50 baht/ QALYs, 31,211.35 baht/QALYs and 31,223.34 baht per QALYs respectively.
Conclusion: Therefore, levofloxacin-PPI based triple therapy was found to be the most cost-effective regimen and the dominant strategy compared with concomitant-PPI or vonoprazan-based triple therapy. It provided higher QALYs and lower treatment costs. Levofloxacin-PPI based triple therapy should be the first choice of an alternative strategy in treating clarithromycin-resistant H. pylori. The results of this study can be used by policymakers to help inform their decisions.
References
2. Phukpo W. The diagnostic test of bloodstream infection for Helicobacter pylori. Tham-Lab. 2018;5(3):3-8.
3. The Gastroenterological Association of Thailand. Thailand Consensus on Helicobacter Pylori Management 2015. Bangkok: Concept Medicus Co.,Ltd; 2015. 1-35 p.
4. Jaka H, Mueller A, Kasang C, Mshana SE. Predictors of triple therapy treatment failure among H. pylori infected patients attending at a tertiary hospital in Northwest Tanzania: a prospective study. BMC Infect Dis. 2019;19(1):447.
5. Vilaichone RK, Quach DT, Yamaoka Y, Sugano K, Mahachai V. Prevalence and Pattern of Antibiotic Resistant Strains of Helicobacter Pylori Infection in ASEAN. Asian Pac J Cancer P. 2018;19(5):1411-3.
6. Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infectionthe Maastricht V/Florence Consensus Report. Gut. 2017;66(1): 6-30.
7. Mahachai V, Vilaichone RK, Pittayanon R, Rojborwonwitaya J, Leelakusolvong S, Maneerattanaporn M, et al. Helicobacterpylori management in ASEAN: The Bangkok consensus report. J Gastroenterol Hepatol. 2018;33(1):37-56.
8. Sachs G, Scott DR, Wen Y. Gastric infection by Helicobacter pylori. Curr Gastroenterol Rep. 2011;13(6):540-6.
9. Tantiwit J. Vonoprazan: The new acid blocker. Center for Continuing Pharmaceutical Education. 2020:1-23.
10. Akazawa Y, Fukuda D, Fukuda Y. Vonoprazan-based therapy for Helicobacter pylori eradication: experience and clinical evidence. Ther Adv Gastroenter. 2016;9(6):845-52.
11. Li M, Oshima T, Horikawa T, Tozawa K, Tomita T, Fukui H, et al. Systematic review with meta-analysis: Vonoprazan, a potent acid blocker, is superior to proton-pump inhibitors for eradication of clarithromycin-resistant strains of Helicobacterpylori. Helicobacter. 2018;23(4):e12495.
12. The Subcommittee of National List of Essential Medicines Development. The Manual of Health Technology Assessment in Thailand. Nonthaburi: The Graphico Systems Co., Ltd.; 2009.
13. Suzuki S, Gotoda T, Kusano C, Iwatsuka K, Moriyama M. The Efficacy and Tolerability of a Triple Therapy Containing a Potassium-Competitive Acid Blocker Compared With a 7-Day PPI-Based Low-Dose Clarithromycin Triple Therapy. Am J Gastroenterol. 2016;111(7):949-56. 822 Volume 73, No.12: 2021 Siriraj Medical Journal https://he02.tci-thaijo.org/index.php/sirirajmedj/index
14. Sanglutong L, Aumpan N, Pornthisarn B, Chonprasertsuk S, Siramolpiwat S, Bhanthumkomol P, et al. Ineffectiveness of 14-Day Vonoprazan-Based Dual Therapy and Vonoprazan-Based Triple Therapy for Helicobacter Pylori Eradication in Area of High Clarithromycin Resistance: A Prospective Randomized Study. Gastroenterology. 2020;158(6):S-571.
15. Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, doubleblind study. Gut. 2016;65(9):1439-46.
16. Kim SY, Chung J-W. Best Helicobacter pylori Eradication Strategy in the Era of Antibiotic Resistance. Antibiotics. 2020; 9(8):436.
17. Maruyama M, Tanaka N, Kubota D, Miyajima M, Kimura T, Tokutake K, et al. Vonoprazan-Based Regimen Is More Useful than PPI-Based One as a First-Line Helicobacter pylori Eradication: A Randomized Controlled Trial. Can J Gastroenterol Hepatol. 2017;2017:4385161-.
18. Tai W-C, Lee C-H, Chiou S-S, Kuo C-M, Kuo C-H, Liang C-M, et al. The Clinical and Bacteriological Factors for Optimal
Levofloxacin-Containing Triple Therapy in Second-Line Helicobacter pylori Eradication. Plos One. 2014;9(8):e105822.
19. Chung JW, Han JP, Kim KO, Kim SY, Hong SJ, Kim TH, et al. Ten-day empirical sequential or concomitant therapy is more effective than triple therapy for Helicobacter pylori eradication: A multicenter, prospective study. Dig Liver Dis. 2016;48(8):888-92.
20. Kim BJ, Lee H, Lee YC, Jeon SW, Kim GH, Kim H-S, et al. Ten-Day Concomitant, 10-Day Sequential, and 7-Day Triple Therapy as First-Line Treatment for Helicobacter pylori Infection: A Nationwide Randomized Trial in Korea. Gut Liver. 2019;13(5):531-40.
21. Park SM, Kim JS, Kim BW, Ji JS, Choi H. Randomized clinical trial comparing 10- or 14-day sequential therapy and 10- or 14-day concomitant therapy for the first line empirical treatment of Helicobacter pylori infection. J Gastroenterol Hepatol. 2017;32(3):589-94.
22. Kim SY, Lee SW, Choe JW, Jung SW, Hyun JJ, Jung YK, et al. Helicobacter pylori eradication rates of concomitant and sequential therapies in Korea. Helicobacter. 2017;22(6).
23. Liou JM, Fang YJ, Chen CC, Bair MJ, Chang CY, Lee YC, et al. Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2016;388(10058):2355-65.
24. Heo J, Jeon SW, Jung JT, Kwon JG, Lee DW, Kim HS, et al. Concomitant and hybrid therapy for Helicobacter pylori infection: A randomized clinical trial. J Gastroenterol Hepatol. 2015;30(9):1361-6.
25. Heo J, Jeon SW, Jung JT, Kwon JG, Kim EY, Lee DW, et al. A randomised clinical trial of 10-day concomitant therapy and standard triple therapy for Helicobacter pylori eradication. Dig Liver Dis. 2014;46(11):980-4.
26. Kim SJ, Chung J-W, Woo HS, Kim SY, Kim JH, Kim YJ, et al. Two-week bismuth-containing quadruple therapy and concomitant therapy are effective first-line treatments for Helicobacter pylori eradication: A prospective open-label randomized trial. World J Gastroenterol. 2019;25(46):6790-8.
27. Peura DA, LaMon JT, Moynihan LK, Bonis PAL. Helicobacter Pylori: Bay Area Houston Gastroenterology Associates; [cited 2020 6 Jun]. Available from: http://www.bayareahoustongastro.com/wp-content/uploads/Helicobacter-Pylori.pdf.
28. Health Information System Development Office. Trend of health in Thailand 2017 [cited 2020 6 Nov]. Available from: https://www.hiso.or.th/health/data/html/search1.php?menu=1&i=.
29. Chen Y, Segers S, Blaser MJ. Association between Helicobacter pylori and mortality in the NHANES III study. Gut. 2013;62(9):1262-9.
30. Apostolopoulos P, Koumoutsos I, Ekmektzoglou K, Dogantzis P, Vlachou E, Kalantzis C, et al. Concomitant versus sequential therapy for the treatment of Helicobacter pylori infection: a Greek randomized prospective study. Scand J Gastroentero. 2016;51(2):145-51.
31. Burden of Disease Thailand. Health Adjusted Life Expectancy: HALE. Burden of Disease Research Program Thailand; 2018.
32. Vilaichone RK, Wongcha Um A, Chotivitayatarakorn P. Low Re-infection Rate of Helicobacter pylori after Successful Eradication in Thailand: A 2 Years Study. Asian Pac J Cancer P. 2017;18(3):695-7.
33. Tamaki H, Noda T, Morita M, Omura A, Kubo A, Ogawa C, et al. An Open-Label, Multi-Center, Randomized, Superiority Trial of Vonoprazan Versus Esomeprazole as Part of First-Line Triple Therapy for Helicobacter Pylori Infection. Gastroenterology. 2019;156(6):S-89.
34. Ang TL, Fock KM, Song M, Ang D, Kwek AB, Ong J, et al. Ten-day triple therapy versus sequential therapy versus concomitant therapy as first-line treatment for Helicobacter pylori infection. J Gastroenterol Hepatol. 2015;30(7):1134-9.
35. The drug’s median price in Thailand [Internet]. National Drug Information. 2020 [cited 20 Apr 2020]. Available from: http://ndi.fda.moph.go.th/drug_value.
36. Drugs price list [Internet]. Department of Internal Trade, Ministry of Commerce. 2020 [cited 9 Dec 2020]. Available from: https://hospitals.dit.go.th/app/drug_price_list.php.
37. Specific screening program (H. pylori) [Internet]. Yanhee Hospital. 2020 [cited 6 Nov 2020]. Available from: https://th.yanhee.net/.
38. Urea Breath Test [Internet]. Bumrungrad International Hospital. 2020 [cited 6 Nov 2020]. Available from: https://www.bumrungrad.
com/en/treatments/urea-breath-test. 39. Urea Breath Test [Internet]. Vichaivej Nongkhaem International Hospital. [cited 6 Nov 2020]. Available from: https://vichaivejnongkhaem.com/packages/ตรวจหาเชื้อ-h-pylori-ด้วยวิธีเป/.
40. Ministry of Public Health, Thailand. The service charge of public health services affiliated with the Ministry of Public Health, Thailand. In: Ministry of Public Health, Thailand, editors.: Ministry of Public Health, Thailand; 2019. p. 4-88.
41. Standard Cost Lists for Health Technology Assessment [Internet]. The Ministry of Public Health, Thailand. 2020 [cited 6 Nov 2020]. Available from: https://costingmenu.hitap.net/.
42. Xie F, Luo N, Lee H-P. Cost effectiveness analysis of populationbased serology screening and (13)C-Urea breath test for Helicobacter pylori to prevent gastric cancer: a markov model. World J Gastroentero. 2008;14(19):3021-7.
43. Grunberg SM, Magnan WF, Herndon J, Naughton ML, Blackwell KL, Wood ME, et al. Determination of utility scores for control of chemotherapy-induced nausea or vomiting. J Support Oncol. 2009;7:W17-W22.
44. Shlomai A, Leshno M, Goldstein DA. Regorafenib treatment for patients with hepatocellular carcinoma who progressed on Lertsirimunkong et al.
https://he02.tci-thaijo.org/index.php/sirirajmedj/index Volume 73, No.12: 2021 Siriraj Medical Journal 823 Original Article SMJ
sorafenib-A cost-effectiveness analysis. Plos One. 2018;13(11):e0207132.
45. Sher DJ, Tishler RB, Pham NL, Punglia RS. Cost-Effectiveness Analysis of Intensity Modulated Radiation Therapy Versus Proton Therapy for Oropharyngeal Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys. 2018;101(4):875-82.
46. Consumer Price Index [Internet]. 2021 [cited 15 February 2021]. Available from: http://www.price.moc.go.th/price/cpi/index_new.asp.
47. Yamasaki T, Owari M, Tokai Y, Amano Y, Nakashima H, Sakaki N, et al. High Dose CAM With Vonoprazan (P-CAB) PLUS AMX Regimen Is the Strongest H. pylori Eradication Triple Therapy Regimens to Improve Success Rate of CAM Based Regimens. Gastroenterology. 2016;150(4, Supplement 1):S879.
48. Areesinpitak T, Kanjanawart S, Nakkam N, Tassaneeyakul W, Vannaphasaht S. Prevalence of CYP2C19, CYP3A4 and FMO3 genetic polymorphisms in healthy northeastern Thai volunteers. Scienceasia. 2020;46(3):397-402.
49. Chuensuwan R. The Novel Drug for Acid Related Diseases. J Prapokklao Hosp Clin Med Educat Center. 2020;37(4):364-72.
Published
How to Cite
Issue
Section
License
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














