Magnetic Resonance Hippocampal Subfield Volumetric Analysis for Differentiating among Healthy Older Adults and Older Adults with Mild Cognitive Impairment or Major Depressive Disorder
DOI:
https://doi.org/10.33192/Smj.2021.102Keywords:
Magnetic resonance hippocampal subfield volumetric analysis, mild cognitive impairment (MCI), major depressive disorder (MDD), healthy older adults (HOA)Abstract
Objective: Depression among older adults is frequently an early symptom of cognitive decline, and is believed to be a risk factor for Alzheimer’s disease (AD). Hippocampal subfield volume loss is found in both mild cognitive impairment (MCI) and major depressive disorder (MDD). We aimed to investigate the potential of MR hippocampal subfield volumetry for discriminating among healthy older adults (HOA) and older adults with MCI or MDD.
Materials and Methods: Seventy age-matched subjects (29 non-depressed MCI, 12 MDD, and 29 HOA) underwent 3-Tesla MR imaging (MRI) with high-resolution 3D-T1W-TFE whole brain. Hippocampal subfield volumetric measurements were performed using FreeSurfer software to distinguish among MCI, MDD, and HOA. Subgroup analysis with amyloid PET result was also performed.
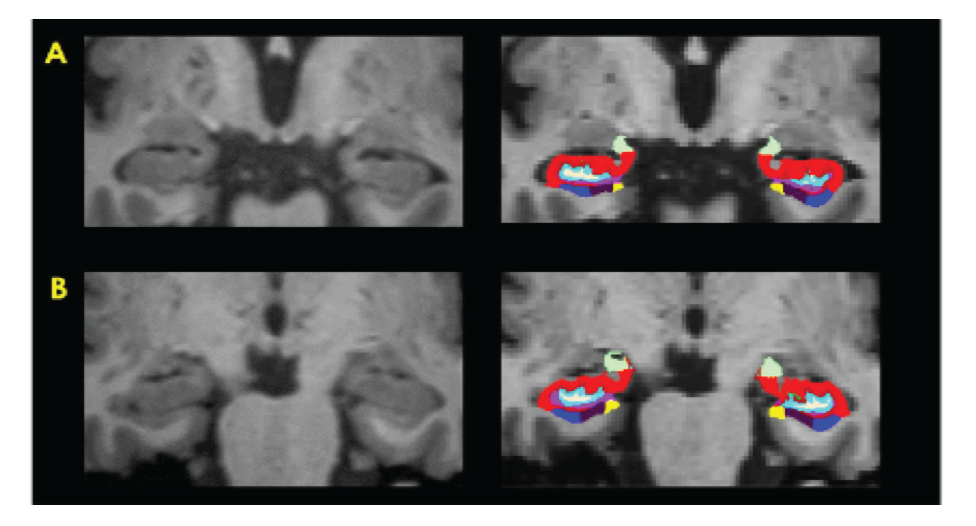
Results: Significantly smaller bilateral hippocampal tail volume was observed in MCI compared to HOA (p=0.004 and p=0.04 on the left and right side, respectively). The same comparative finding was observed at left HATA (hippocampus-amygdala-transition-area) of MCI (p=0.046). Other regions showed non-significantly smaller size in MCI than in HOA [left molecular layer HP (p=0.06), left whole hippocampus (p=0.06), and left CA1 (p=0.07)]. There was a non-significant trend toward smaller size in almost all 13 subfield hippocampal regions of MCI compared to MDD, even in subgroup analysis with amyloid PET result.
Conclusion: MR hippocampal subfield volumetry may have value in routine clinical practice for screening individuals with MCI, and may be a valuable adjunct to amyloid PET study for very early-stage diagnosis of AD.
References
2. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer Dement. 2011;7(3):270-9.
3. Korczyn AD, Halperin I. Depression and dementia. J Neurol Sci. 2009;283(1-2):139-42.
4. Palazidou E. The neurobiology of depression. Vol. 101, British Medical Bulletin. 2012. p.127-45.
5. Khan W, Westman E, Jones N, Wahlund LO, Mecocci P, Vellas B, et al. Automated Hippocampal Subfield Measures as Predictors Wongsawaeng et al. https://he02.tci-thaijo.org/index.php/sirirajmedj/index Volume 73, No.12: 2021 Siriraj Medical Journal 807 Original Article SMJ of Conversion from Mild Cognitive Impairment to Alzheimer’s Disease in Two Independent Cohorts. Brain Topogr. 2015;28(5):746-59.
6. Mckinnon MC, Yucel K, Nazarov A, Macqueen GM, Mckinnon P. Presented as a poster at the 58th Annual Conference of the Canadian Psychiatric Assocation. J Psychiatry Neurosci. 2009;34:
7. Muangpaisan W, Md A, Sitthichai Bsc K, Richardson Phd K, Brayne C. The Distribution of Thai Mental State Examination Scores among Non-Demented Elderly in Suburban Bangkok Metropolitan and Associated Factors. J Med Assoc Thai. 2015; 98(9):916-24.
8. Lotrakul M, Sukanich P, Sukying C. The Reliability and Validity of Thai version of Hamilton Rating Scale for Depression. J Psychiat Assoc Thai. 1996;41(4):235-46.
9. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. American Psychiatric Association; 2013.
10. Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117-37.
11. Westman E, Aguilar C, Muehlboeck JS, Simmons A. Regional magnetic resonance imaging measures for multivariate analysis in Alzheimer’s disease and mild cognitive impairment. Brain Topogr. 2013;26(1):9-23.
12. Thientunyakit T, Sethanandha C, Muangpaisan W, Chawalparit O, Arunrungvichian K, Siriprapa T, et al. Implementation of [18F]-labeled amyloid brain PET imaging biomarker in the diagnosis of Alzheimer’s disease. Nucl Med Commun. 2018; 39(2):186-92.
13. ADNI-GO PET Technical Procedures Manual for FDG and AV-45 ADNI-GO PET Technical Procedures Manual, 2011.
14. ADNI 2 PET Technical Procedures Manual for FDG and AV-45 ADNI 2 PET Technical Procedures Manual AV-45 (Florbetapir F 18) & FDG. 2011.
15. Minoshima S, Drzezga AE, Barthel H, Bohnen N, Djekidel M, Lewis DH, et al. SNMMI procedure standard/EANM practice guideline for amyloid PET imaging of the brain 1.0. J Nucl Med. 2016;57(8):1316–22.
16. Fanselow MS, Dong HW. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron. 2010;65(1):7-19.
17. Samuels BA, Hen R. Neurogenesis and affective disorders. Eur J Neurosci. 2011;33(6):1152-9.
18. Scharfman HE, Chao MV. The entorhinal cortex and neurotrophin signaling in Alzheimer’s disease and other disorders. Cogn Neurosci. 2013;4(3-4):123-35.
19. Samuels BA, Leonardo ED, Hen R. Hippocampal subfields and major depressive disorder. Vol. 77, Biological Psychiatry. Elsevier USA; 2015. p. 210-1.
20. Katsuki A, Watanabe K, Nguyen L, Otsuka Y, Igata R, Ikenouchi A, et al. Structural changes in hippocampal subfields in patients with continuous remission of drug-naive major depressive disorder. Int J Mol Sci. 2020;21(9):3032.
21. Han KM, Kim A, Kang W, Kang Y, Kang J, Won E, et al. Hippocampal subfield volumes in major depressive disorder and bipolar disorder. Eur Psychiatry. 2019;57:70-77.
Published
How to Cite
Issue
Section
License
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














