Prevalence and Factors Associated with the Loss of PTEN Expression in Patients with Lung Cancer
DOI:
https://doi.org/10.33192/Smj.2022.7Keywords:
PTEN, immunohistochemistry, tumor infiltrating lymphocytes, lung cancerAbstract
Background: Phosphatase and tensin homolog (PTEN) is a major tumor suppressor gene and is involved in cell survival control. PTEN loss of expression (PTEN-) is associated with a poor outcome. Our study investigated the prevalence of PTEN- in terms of its characteristics and disease prognosis for lung cancer patients.
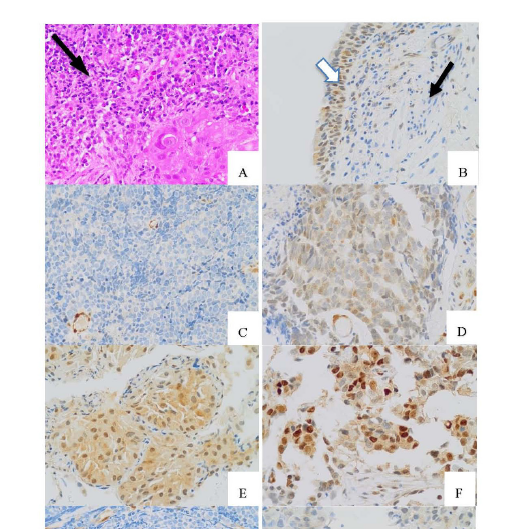
Materials and Methods: In total, 167 tissue blocks from lung cancer patients at Chareonkrung Pracharak Hospital between January 2010 and December 2020 were studied through immunohistochemistry staining (IHC) for PTEN expression. The clinicopathological factors, IHC features, and epidermal growth factor receptor (EGFR) status were analyzed in association with PTEN- in term of prognosis and the overall survival (OS).
Result: Adenocarcinoma was the major subtype (85.6%) and most patients (90.6%) were diagnosed at stage IV of lung cancer. The prevalence of PTEN- was 66.5%. A location at the left lower lobe (LLL) location and the absence of tumor-infiltrating lymphocytes (TILs) were significantly associated with PTEN- (p=0.039, p=0.046), while the smoking was likely correlated but not statistically significant (p=0.09). The median OS for PTEN- was not significantly different from PTEN+ (8.88 vs 7.20 months, p=0.38). However, smoking, Eastern cooperative oncology group (ECOG) status and primary symptoms were significantly associated with poorer OS.
Conclusion: The prevalence of PTEN- was higher in our studies. Absent TILs and a LLL location were independent factors associated with PTEN-. However, a right upper lobe (RUL) location with PTEN- tended to have a poor prognosis. Interestingly, better survival was found in active smokers with PTEN-. Further survival studies in cases with no TILs lesions and active smokers in associations PTEN expression and other immune-related biomarkers, such as programmed death–ligand 1 (PD-L1), are warranted.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30.
Hospital-based cancer registry 2015. Pornsup Printing Co., LTD: National Cancer Institute, Department of medical services, Ministry of Public Health, Thailand.; 2017. 72 p.
David Planchard SN, Solange Peters, Raffaele Califano, Jean-Yves Douillard, Francesca Longo,. Non-small-cell lung cancer (NSCLC) An ESMO guide for patients. Via Ginevra 4 6900 Lugano Switzerland: European Society for Medical Oncology (ESMO); 2019. 64 p.
Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5(3):288-300.
Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10(9):1243-60.
Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J, et al. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci U S A. 1999;96(11):6199-204.
Kang YH, Lee HS, Kim WH. Promoter methylation and silencing of PTEN in gastric carcinoma. Lab Invest. 2002;82(3):285-91.
Serra H, Chivite I, Angulo-Urarte A, Soler A, Sutherland JD, Arruabarrena-Aristorena A, et al. PTEN mediates Notch-dependent stalk cell arrest in angiogenesis. Nat Commun. 2015;6:7935.
Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015;16(2):178-87.
Jo HS, Kang KH, Joe CO, Kim JW. Pten coordinates retinal neurogenesis by regulating Notch signalling. EMBO J. 2012;31(4):817-28.
Tang JM, He QY, Guo RX, Chang XJ. Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer. 2006;51(2):181-91.
A. Soltermann UR, O. Dafni, E. Verbeken, E. Thunnissen, A. Warth, R. Cheney,. Prevalence and clinical associations of PTEN loss in non-small cell lung carcinoma (NSCLC) patients (pts) of the European Thoracic Oncology Platform (ETOP) Lungscape cohort. Annals of Oncology. 2016;27:vi526–vi44.
Seol-Bong YOO XX, Hyun-Ju LEE, Sanghoon JHEON, Choon-Taek LEE, Gheeyoung CHOE, Jin-Haeng CHUNG. Loss of PTEN Expression is an Independent Poor Prognostic Factor in Non-small Cell Lung Cancer. Korean Journal of Pathology. 2011:329-35.
Akca H, Demiray A, Tokgun O, Yokota J. Invasiveness and anchorage independent growth ability augmented by PTEN inactivation through the PI3K/AKT/NFkB pathway in lung cancer cells. Lung Cancer. 2011;73(3):302-9.
Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999;96(8):4240-5.
Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100(4):387-90.
Zhao Y, Zheng R, Li J, Lin F, Liu L. Loss of phosphatase and tensin homolog expression correlates with clinicopathological features of non-small cell lung cancer patients and its impact on survival: A systematic review and meta-analysis. Thorac Cancer. 2017;8(3):203-13.
Forgacs E, Biesterveld EJ, Sekido Y, Fong K, Muneer S, Wistuba, II, et al. Mutation analysis of the PTEN/MMAC1 gene in lung cancer. Oncogene. 1998;17(12):1557-65.
Cui M, Augert A, Rongione M, Conkrite K, Parazzoli S, Nikitin AY, et al. PTEN is a potent suppressor of small cell lung cancer. Mol Cancer Res. 2014;12(5):654-9.
Noro R, Gemma A, Miyanaga A, Kosaihira S, Minegishi Y, Nara M, et al. PTEN inactivation in lung cancer cells and the effect of its recovery on treatment with epidermal growth factor receptor tyrosine kinase inhibitors. Int J Oncol. 2007;31(5):1157-63.
Sos ML, Koker M, Weir BA, Heynck S, Rabinovsky R, Zander T, et al. PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer Res. 2009;69(8):3256-61.
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259-71.
Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, et al. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci U S A. 1999;96(5):2110-5.
Xiao J, Hu CP, He BX, Chen X, Lu XX, Xie MX, et al. PTEN expression is a prognostic marker for patients with non-small cell lung cancer: a systematic review and meta-analysis of the literature. Oncotarget. 2016;7(36):57832-40.
Horton BL, Williams JB, Cabanov A, Spranger S, Gajewski TF. Intratumoral CD8(+) T-cell Apoptosis Is a Major Component of T-cell Dysfunction and Impedes Antitumor Immunity. Cancer Immunol Res. 2018;6(1):14-24.
Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717-34.
Hlaing AM, Furusato B, Udo E, Kitamura Y, Souda M, Masutani M, et al. Expression of phosphatase and tensin homolog and programmed cell death ligand 1 in adenosquamous carcinoma of the lung. Biochem Biophys Res Commun. 2018;503(4):2764-9.
Soria JC, Lee HY, Lee JI, Wang L, Issa JP, Kemp BL, et al. Lack of PTEN expression in non-small cell lung cancer could be related to promoter methylation. Clin Cancer Res. 2002;8(5):1178-84.
Spoerke JM, O'Brien C, Huw L, Koeppen H, Fridlyand J, Brachmann RK, et al. Phosphoinositide 3-kinase (PI3K) pathway alterations are associated with histologic subtypes and are predictive of sensitivity to PI3K inhibitors in lung cancer preclinical models. Clin Cancer Res. 2012;18(24):6771-83.
Stjernstrom A, Karlsson C, Fernandez OJ, Soderkvist P, Karlsson MG, Thunell LK. Alterations of INPP4B, PIK3CA and pAkt of the PI3K pathway are associated with squamous cell carcinoma of the lung. Cancer Med. 2014;3(2):337-48.
Yanagawa N, Leduc C, Kohler D, Saieg MA, John T, Sykes J, et al. Loss of phosphatase and tensin homolog protein expression is an independent poor prognostic marker in lung adenocarcinoma. J Thorac Oncol. 2012;7(10):1513-21.
Goncharuk VN, del-Rosario A, Kren L, Anwar S, Sheehan CE, Carlson JA, et al. Co-downregulation of PTEN, KAI-1, and nm23-H1 tumor/metastasis suppressor proteins in non-small cell lung cancer. Ann Diagn Pathol. 2004;8(1):6-16.
David Planchard SN, Solange Peters, Raffaele Califano, Jean-Yves Douillard, Francesca Longo. ESMO Non-small-cell lung cancer (NSCLC) An ESMO guide for patients. Via Ginevra 4 6900 Lugano Switzerland: European Society for Medical Oncology (ESMO); 2019. 64 p.
Cancer Fact sheet [Internet]. National Cancer Institute. 2021 [cited 07.20.2021].
Wang J, Chen H, Liao Y, Chen N, Liu T, Zhang H, et al. Expression and clinical evidence of miR-494 and PTEN in non-small cell lung cancer. Tumour Biol. 2015;36(9):6965-72.
Wang L, Yue W, Zhang L, Zhao X, Wang Y, Xu S. mTOR and PTEN expression in non-small cell lung cancer: analysis by real-time fluorescence quantitative polymerase chain reaction and immunohistochemistry. Surg Today. 2012;42(5):419-25.
Kim HS, Kim GY, Lim SJ, Kim YW. Expression of the mammalian target of rapamycin pathway markers in lung adenocarcinoma and squamous cell carcinoma. Pathobiology. 2012;79(2):84-93.
Yoshizawa A, Fukuoka J, Shimizu S, Shilo K, Franks TJ, Hewitt SM, et al. Overexpression of phospho-eIF4E is associated with survival through AKT pathway in non-small cell lung cancer. Clin Cancer Res. 2010;16(1):240-8.
Lim WT, Zhang WH, Miller CR, Watters JW, Gao F, Viswanathan A, et al. PTEN and phosphorylated AKT expression and prognosis in early- and late-stage non-small cell lung cancer. Oncol Rep. 2007;17(4):853-7.
Shin E, Choi CM, Kim HR, Jang SJ, Park YS. Immunohistochemical characterization of the mTOR pathway in stage-I non-small-cell lung carcinoma. Lung Cancer. 2015;89(1):13-8.
Xiu-Jun Chang X-SZ, Zi-Tong Wang, Fu-Gen Li, Yong Duan, Ming Han. The clinical significance of loss of FHIT and PTEN expression in 289 patients with non-small-cell lung cancer. Transl Lung Cancer Res. 2016;5:294–301.
Yoo SB, Kim YJ, Kim H, Jin Y, Sun PL, Jheon S, et al. Alteration of the E-cadherin/beta-catenin complex predicts poor response to epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) treatment. Ann Surg Oncol. 2013;20 Suppl 3:S545-52.
Scrima M, De Marco C, Fabiani F, Franco R, Pirozzi G, Rocco G, et al. Signaling networks associated with AKT activation in non-small cell lung cancer (NSCLC): new insights on the role of phosphatydil-inositol-3 kinase. PLoS One. 2012;7(2):e30427.
Port JL, Kent MS, Korst RJ, Libby D, Pasmantier M, Altorki NK. Tumor size predicts survival within stage IA non-small cell lung cancer. Chest. 2003;124(5):1828-33.
Franceschini JP, Jamnik S, Santoro IL. Survival in a cohort of patients with lung cancer: the role of age and gender in prognosis. J Bras Pneumol. 2017;43(6):431-6.
Li X, Yang Y, Zhang H, Yue W, Zhang T, Lu B, et al. High levels of Phosphatase and Tensin Homolog Expression Predict Favorable Prognosis in Patients with Non-small Cell Lung Cancer. Cell Biochem Biophys. 2015;73(3):631-7.
Ji Y, Zheng M, Ye S, Chen J, Chen Y. PTEN and Ki67 expression is associated with clinicopathologic features of non-small cell lung cancer. J Biomed Res. 2014;28(6):462-7.
An SJ, Lin QX, Chen ZH, Su J, Cheng H, Xie Z, et al. Combinations of laminin 5 with PTEN, p-EGFR and p-Akt define a group of distinct molecular subsets indicative of poor prognosis in patients with non-small cell lung cancer. Exp Ther Med. 2012;4(2):226-30.
Wang C, Yang R, Yue D, Zhang Z. Expression of FAK and PTEN in bronchioloalveolar carcinoma and lung adenocarcinoma. Lung. 2009;187(2):104-9.
Zolota VG, Tzelepi VN, Leotsinidis M, Zili PE, Panagopoulos ND, Dougenis D, et al. Histologic-type specific role of cell cycle regulators in non-small cell lung carcinoma. J Surg Res. 2010;164(2):256-65.
Regina S, Valentin JB, Lachot S, Lemarie E, Rollin J, Gruel Y. Increased tissue factor expression is associated with reduced survival in non-small cell lung cancer and with mutations of TP53 and PTEN. Clin Chem. 2009;55(10):1834-42.
Jin G, Kim MJ, Jeon HS, Choi JE, Kim DS, Lee EB, et al. PTEN mutations and relationship to EGFR, ERBB2, KRAS, and TP53 mutations in non-small cell lung cancers. Lung Cancer. 2010;69(3):279-83.
Kohnoh T, Hashimoto N, Ando A, Sakamoto K, Miyazaki S, Aoyama D, et al. Hypoxia-induced modulation of PTEN activity and EMT phenotypes in lung cancers. Cancer Cell Int. 2016;16:33.
Lee HW, Park YS, Park S, Lee CH. Poor prognosis of NSCLC located in lower lobe is partly mediated by lower frequency of EGFR mutations. Sci Rep. 2020;10(1):14933.
Schalper KA, Brown J, Carvajal-Hausdorf D, McLaughlin J, Velcheti V, Syrigos KN, et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst. 2015;107(3).
Gao G, Wang Z, Qu X, Zhang Z. Prognostic value of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: a systematic review and meta-analysis. BMC Cancer. 2020;20(1):179.
Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14(5):1291-5.
Published
How to Cite
Issue
Section
License
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














