Stability and Sterility of Extemporaneously Prepared 0.01% Atropine Ophthalmic Solution in Artificial Tears and Balanced Salt Solution
DOI:
https://doi.org/10.33192/Smj.2022.12Keywords:
Myopia, atropine, stability, sterility, artificial tear, balanced salt solutionAbstract
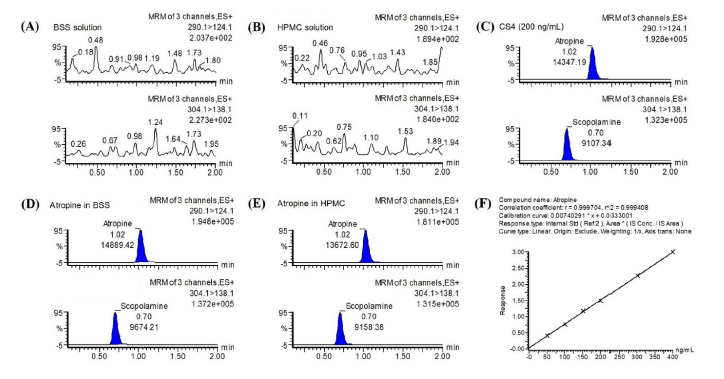
Objective: The aim of this study was to investigate the physicochemical and microbiological stability of extemporaneously prepared 0.01% atropine ophthalmic solution in unopened eyedropper and in simulated use condition.
Materials and Methods: Two formulations of 0.01% atropine solutions, atropine in artificial tear and atropine in balanced salt solution (BSS), were prepared using 0.5 mL insulin syringes. In unopened conditions, 0.01% atropine solutions were stored for six months at refrigerated temperature (2-8°C) or room temperature (25±2°C). Visual inspection, atropine quantification, pH measurements, and sterility assay were analyzed at baseline, and every month for six months. In simulated use condition, 0.01% atropine solutions stored at refrigerated and room temperature were analyzed at 0, 15 and 30 days.
Results: In unopened conditions, both of 0.01% atropine formulations stored at refrigerated temperature showed satisfactory stability. Atropine remained within 90% to 110% of the initial concentration up to six months. Under room temperature, both formulations of atropine were less than 90% of their initial value after 4 months storage. In simulated use condition, atropine concentration was within 90% to 110% of initial value after 30 days at refrigerated and room temperature. All atropine solutions prepared in artificial tear and BSS were free from bacterial contamination throughout the study. No alteration of physical appearance (i.e., precipitation, discoloration) was observed, and pH values also remained nearly unchanged.
Conclusion: Both formulations of 0.01% atropine are physicochemically stable for up to 6 months when kept unopened in refrigerator, and for 1 month at refrigerated and room temperatures in simulated use condition.
References
Foster Pa, Jiang Y. Epidemiology of myopia. Eye. 2014;28(2):202-08.
WHO. The Impact of Myopia and High Myopia: Report of the Joint World Health Organization-Brien Holden Vision Institute Global Scientific Meeting on Myopia. University of New South Wales Sydney, Australia; 2017.
Ding B-Y, Shih Y-F, Lin LL, Hsiao CK, Wang I-J. Myopia among schoolchildren in East Asia and Singapore. Survey of Ophthalmology. 2017;62(5):677-97.
Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-42.
Cheng J, Yang Y, Kong X, Zeng L, Chen Z, Xu J, et al. The Effect of 0.01% Atropine Eye Drops on the Ocular Surface in Children for the Control of Myopia-The Primary Results from a Six-Month Prospective Study. Therapeutics and Clinical Risk Management. 2020;16:735-40.
Sankaridurg P, Conrad F, Tran H, Zhu J. Controlling progression of myopia: optical and pharmaceutical strategies. The Asia-Pacific Journal of Ophthalmology. 2018;7(6):405-14.
Tran HD, Tran YH, Tran TD, Jong M, Coroneo M, Sankaridurg P. A review of myopia control with atropine. J Ocul Pharmacol Ther. 2018;34(5):374-79.
Tran H, Ha T. Mechanism of Action of Atropine in Controlling Myopia Progression. 2020.
Ruan Y, Patzak A, Pfeiffer N, Gericke A. Muscarinic Acetylcholine Receptors in the Retina-Therapeutic Implications. Int J Mol Sci. 2021;22(9):4989.
Chua W-H, Balakrishnan V, Chan Y-H, Tong L, Ling Y, Quah B-L, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285-91.
Yam JC, Jiang Y, Tang SM, Law AK, Chan JJ, Wong E, et al. Low-concentration atropine for myopia progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126(1):113-24.
International Conference on Harmonisation (ICH). Topic Q2 (R1) Validation of analytical procedures: text and methodology. European Medicines Agency, London; 1995.
International Conference on Harmonisation (ICH). Topic Q 6 A Specifications: test procedures and acceptance criteria for new drug substances and new drug products: chemical substances European Medicines Agency, London; 2000.
Sautou V, Brossard D, Chedru-Legros V, Crauste-Manciet S, Fleury-Souverain S, Lagarce F. Methodological guidelines for stability studies of hospital pharmaceutical preparations. Part 1: liquid preparations. SFPC and GERPAC; 2013.
The United States Pharmacopeia 42-The National Formulary 37. United States Pharmacopeial Convention. Inc., Rockville, MD; 2019.
Saito J, Imaizumi H, Yamatani A. Physical, chemical, and microbiological stability study of diluted atropine eye drops. J Pharm Health Care Sci. 2019;5(1):1-6.
Berton B, Chennell P, Yessaad M, Bouattour Y, Jouannet M, Wasiak M, et al. Stability of Ophthalmic Atropine Solutions for Child Myopia Control. Pharmaceutics. 2020;12(8):781-97.
Farenq P, Jobard M, Cros C, Bezia C, Brandely-Piat M, Batista R, editors. Physical, Chemical and Microbiological Stability Study of 0.1 mg mL−1 Atropine Eye Drops. Proceedings of the 22th European GERPAC Conference, Hyères, France; 2019.
Bullimore MA, Richdale K. Myopia Control 2020: Where are we and where are we heading? Ophthalmic Physiol Opt. 2020 May;40(3):254-270.
Schier JG, Ravikumar PR, Nelson LS, Heller MB, Howland MA, Hoffman RS. Preparing for chemical terrorism: stability of injectable atropine sulfate. Acad Emerg Med. 2004;11(4):329-34.
Iyamu E, Enobakhare O. pH and Osmolality of Pre-corneal Tear Film and Commercially Available Artificial Tears. EC Opthalmology. 2019; 11: 17-25.
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














