Keratinocyte Culture: Siriraj’s Experience
DOI:
https://doi.org/10.33192/Smj.2022.34Keywords:
Keratinocyte culture, keratinocyte culture in Siriraj Hospital, cultured epithelium autograft, CEA, cultured epithelium autograft in Siriraj Hospital, CEA in Siriraj HospitalAbstract
Objective: Cell-based therapy is gaining increasing prominence in medicine, where it has the potential to replace or repair damaged tissue using new engineered cells. Skin cell engineering, also known as keratinocyte culture or cultured epithelial autograft (CEA), is a promising field in cell-based therapy. CEA is now used in many parts of the world as an alternative treatment for some diseases that require large defects to be covered, such as severe and major burn patients and congenital melanocytic nevus. The use of CEA in conjunction with acellular skin substitution is rapidly expanding.
Materials and Methods: This study is an initiative aimed at supporting the production and use of keratinocyte cultures at Siriraj Hospital. This is the first stage of developing sheet keratinocyte culture in vitro.
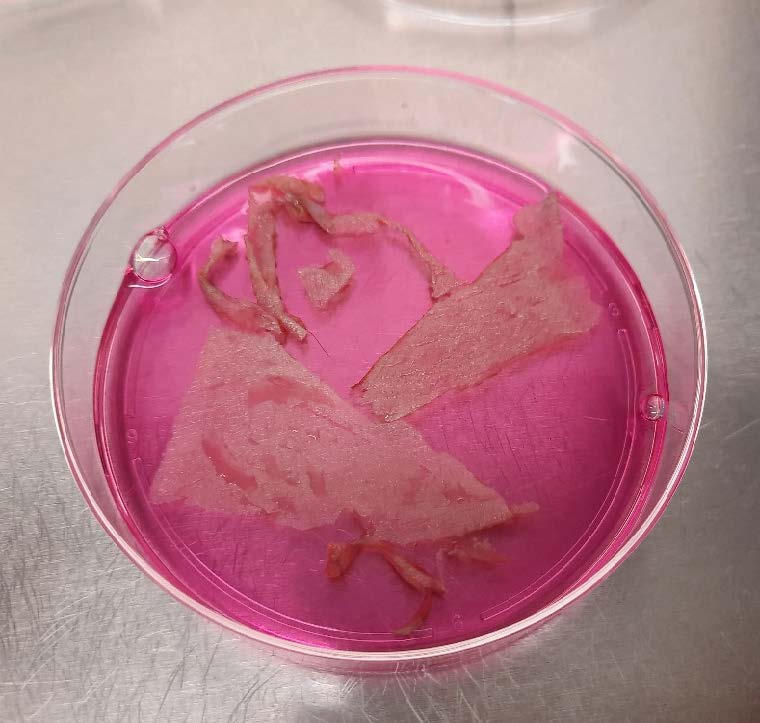
Results: Our study yielded very promising results. As feeder cells, we used irradiated 3T3 murine fibroblasts, as per the standard protocol for keratinocyte culture. The growth duration was four weeks: 2 weeks for the 3T3 murine fibroblasts and 2 weeks for the keratinocytes. The keratinocytes grew rapidly and formed sheets with irradiated 3T3 murine fibroblasts. The retrieval of the cell sheets was straightforward thanks to the temperature-response cell culture dish and halo-ring cell recovery sheet. Flow cytometry revealed that the cells had a very high viability and purity. H&E staining revealed the sheets comprised two to four layers of stratified epithelial tissue.
Conclusion: From this study, our method of manufacturing the CEA can offer a promising result. This can be use in the treatment which require large skin coverage. However, we aim to initiate animal and human trial phase next.
References
Chrapusta A, Nessler MB, Drukala J, Bartoszewicz M, Madry R. A comparative analysis of advanced techniques for skin reconstruction with autologous keratinocyte culture in severely burned children: own experience. Postepy Dermatol Alergol 2014;31(3):164-9. DOI: 10.5114/pdia.2014.43190.
Morimoto N, Kakudo N, Kako A, et al. A case report of the first application of culture epithelial autograft (JACE((R))) for giant congenital melanocytic nevus after its approval in Japan. J Artif Organs 2018;21(2):261-264. DOI: 10.1007/s10047-017-1007-0.
Morimoto N, Jinno C, Sakamoto M, Kakudo N, Yamaoka T, Kusumoto K. An Exploratory Clinical Trial of a Novel Treatment for Giant Congenital Melanocytic Nevi Combining Inactivated Autologous Nevus Tissue by High Hydrostatic Pressure and a Cultured Epidermal Autograft: Study Protocol. JMIR Res Protoc 2016;5(3):e162. DOI: 10.2196/resprot.6195.
Ter Horst B, Chouhan G, Moiemen NS, Grover LM. Advances in keratinocyte delivery in burn wound care. Adv Drug Deliv Rev 2018;123:18-32. DOI: 10.1016/j.addr.2017.06.012.
Green H, Rheinwald JG, Sun TT. Properties of an epithelial cell type in culture: the epidermal keratinocyte and its dependence on products of the fibroblast. Prog Clin Biol Res 1977;17:493-500. (https://www.ncbi.nlm.nih.gov/pubmed/928463).
Rasmussen C, Thomas-Virnig C, Allen-Hoffmann BL. Classical human epidermal keratinocyte cell culture. Methods Mol Biol 2013;945:161-75. DOI: 10.1007/978-1-62703-125-7_11.
Kljenak A, Tominac Trcin M, Bujic M, et al. Fibrin gel as a scaffold for skin substitute - production and clinical experience. Acta Clin Croat 2016;55(2):279-89. DOI: 10.20471/acc.2016.55.02.15.
Gerlach JC, Johnen C, Ottomann C, Bräutigam K, Plettig J, Belfekroun C, et al. Method for autologous single skin cell isolation for regenerative cell spray transplantation with noncultured cells. Int J Artif Organs 2011;34(3):271-9. DOI: 10.5301/ijao.2011.6508.
Ramos MG, Ramos DG, Ramos CG. Evaluation of treatment response to autologous transplantation of noncultured melanocyte/keratinocyte cell suspension in patients with stable vitiligo. An Bras Dermatol 2017;92(3):312-318. DOI: 10.1590/abd1806-4841.20175700.
Holmes JHt, Molnar JA, Shupp JW, Hickerson WL, King BT, Foster KN, et al. Demonstration of the safety and effectiveness of the RECELL((R)) System combined with split-thickness meshed autografts for the reduction of donor skin to treat mixed-depth burn injuries. Burns 2019;45(4):772-82. DOI:10.1016/j.burns.2018.11.002.
Peirce SC, Carolan-Rees G. ReCell((R)) Spray-On Skin System for Treating Skin Loss, Scarring and Depigmentation after Burn Injury: A NICE Medical Technology Guidance. Appl Health Econ Health Policy 2019;17(2):131-41. DOI: 10.1007/s40258-018-00457-0.
Haldar S, Sharma A, Gupta S, Chauhan S, Roy P, Lahiri D. Bioengineered smart trilayer skin tissue substitute for efficient deep wound healing. Mater Sci Eng C Mater Biol Appl 2019;105:110140. DOI: 10.1016/j.msec.2019.110140.
Cubo N, Garcia M, Del Canizo JF, Velasco D, Jorcano JL. 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication 2016;9(1):015006. DOI: 10.1088/1758-5090/9/1/015006.
Horch RE, Wagner G, Bannasch H, Kengelbach-Weigand A, Arkudas A, Schmitz M. Keratinocyte Monolayers on Hyaluronic Acid Membranes as “Upside-Down” Grafts Reconstitute Full-Thickness Wounds. Med Sci Monit 2019;25:6702-6710. DOI: 10.12659/MSM.915649.
Matsumura H, Gondo M, Imai R, Shibata D, Watanabe K. Chronological histological findings of cultured epidermal autograft over bilayer artificial dermis. Burns 2013;39(4):705-13. DOI: 10.1016/j.burns.2012.10.004.
Matsumura H, Matsushima A, Ueyama M, Kumagai N. Application of the cultured epidermal autograft “JACE((R)”) for treatment of severe burns: Results of a 6-year multicenter surveillance in Japan. Burns 2016;42(4):769-76. DOI: 10.1016/j.burns.2016.01.019.
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














