Correlation of Cerebral Atrophy and White Matter Hyperintensity Burden in MRI with Clinical Cognitive Decline
DOI:
https://doi.org/10.33192/Smj.2022.39Keywords:
Cerebral atrophy, hyperintensity, MRI, clinical cognitive declineAbstract
Objective: Dementia is a disease of gradual memory and cognitive loss that affects an individual’s day-to-day activities and is caused by permanent brain damage. Majority of patients are from the elderly population and only
2 to 10 % of affected population is less than 65 years.
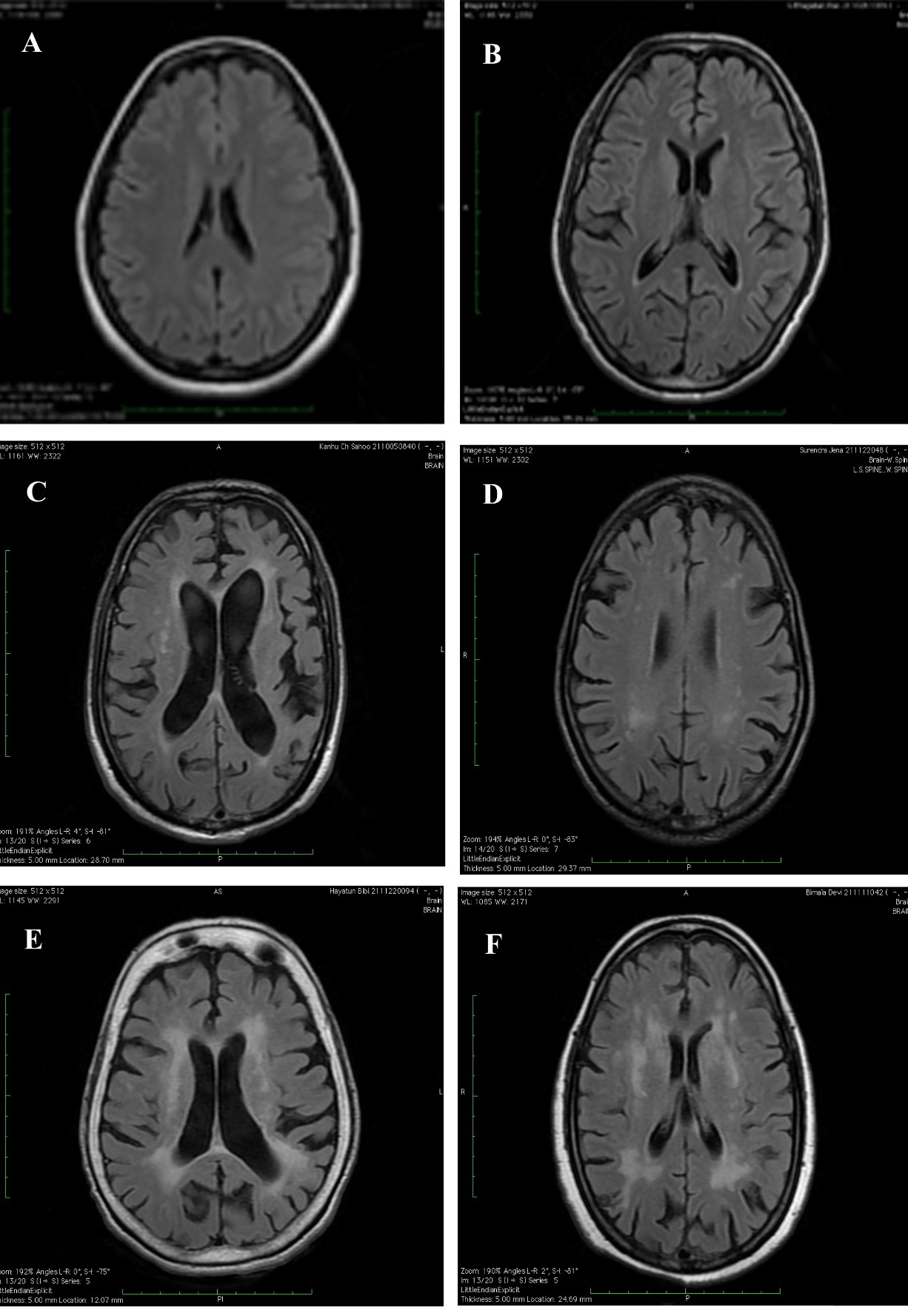
Materials and Methods: We obtained a correlation of severity of white matter hyperintensity (WMH) burden in MRI with severity of clinically assessed cognitive decline. And also analysed the severity of cerebral atrophy in MRI with severity of clinically assessed cognitive decline.
Results: In our study Fazekas scoring for WMHs showed a sensitivity of 87.5% and specificity of 83.3% on correlation with clinical cognitive decline assessed by ADAS-Cog. Also, MTA scale for cerebral atrophy showed a sensitivity of 72% and specificity of 88% on correlation with clinical cognitive decline assessed by ADAS-Cog. Significant P-value have been obtained for both the above visual rating scales of MRI (Fazekas and MTA) by linear regression, on correlation with clinically assessed cognitive decline.
Conclusion: White matter disease assessed by Fazekas scale and cerebral atrophy by MTA scale on MRI brain correlated well with cognitive decline clinically assessed by neuropsychological tests.
References
World Alzheimer Report 2014. Available from: https://www.alz.co.uk/research/WorldAlzheimerReport2014.pdf
Bose A, Shankardass MK. Growing old in India: voices reveal, statistics speak. New Delhi: BR Publishing Corporation; 2000. p.244-6.
Tantanokit T, Bosittipichet T, Leesri T. The Study of Prevalence and Associated Factors of Dementia in the Elderly. Siriraj Med J. 2021;73(4):224-35.
Sukhatunga K, Phattarayuttawat S, Luchom M, Chantra J, Chaiyasit W, Bunnagulrote K. Depression and Dementia in Thai Elderly in Urban and Rural Communities. Siriraj Med J. 1999;51(4):232-43.
Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. 5th ed. New York, NY: Oxford University Press; 2012.
Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med. 2013;29(4):737-52.
Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7(3):268-82.
Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch Neurol. 1999;56(3):338-44.
Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, et al. Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci. 2009;21(2):289-302.
Dennis EL, Thompson PM. Functional brain connectivity using fMRI in aging and Alzheimer’s disease. Neuropsychol Rev. 2014;24(1):49-62.
Pannese E. Morphological changes in nerve cells during normal aging. Brain Struct Funct. 2011;216(2):85-89.
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572-80.
Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology. 1993;43(1):192-7.
Galton CJ, Patterson K, Graham K, Lambon-Ralph MA, Williams G, Antoun N, et al. Differing pattern soft emporal atrophyin Alzheimer’s disease and semantic dementia. Neurology. 2001;57(2):216-25.
Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014;88(4):640-651.
Romero JR, Beiser A, Himali JJ, Shoamanesh A, DeCarli C, Seshadri S. Cerebral microb leed sand risk ofi ncident dementia: the Framingham Heart Study. Neurobiol Aging. 2017;54:94-99.
Graff-Radford J, Simino J, Kantarci K, Mosley TH Jr, Griswold ME, Windham BG, et al. Neuroimaging correlates of cerebral microbleeds: the ARIC Study (Atherosclerosis Risk in Communities). Stroke. 2017;48(11):2964-72.
Poungvarin N, Prayoonwiwat N, Senanarong V, Chaisevikul R, Danchaivijitr C, Nilanont Y. Siriraj Acute Stroke Unit: The Experience of 614 Patients. Siriraj Med J. 2002;54(3):151-8.
Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, et al. Risk factors for dementiaint he cardiovascular health cognition study. Neuroepidemiology. 2003;22(1):13-22.
Barnes DE, Covinsky KE, Whitmer RA, Kuller LH, Lopez OL, Yaffe K. Predicting risk of dementia in older adults: the late-life dementia risk index. Neurology. 2009;73(3):173-79.
Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41(4):600-6.
Knopman DS, Griswold ME, Lirette ST, Gottsman RF, Kantarci K, Sharrett AR, et al. ARIC Neurocognitive Investigators. Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: atherosclerosis risk in communities neurocognitive study. Stroke. 2015;46(2):433-40.
Gottesman RF, Schneider AL, Zhou Y, Coresh J, Green E, Gupta N, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317(14):1443-50.
Chawalparit O, Saenanarong V, Chiewvit P. CT Criteria in Vascular Dementia: A Study in Thai Population. Siriraj Med J. 2006;58(2):644-7.
Barkhof F, Hazewinkel M, Binnewijzend M, Smithuis R. Dementia - Role of MRI updated version. [Internet] Alzheimer Centre and Image Analysis Centre, Vrije Universiteit Medical Center, Amsterdam and the Rijnland Hospital, Leiderdorp, The Netherlands. Available from: https://radiologyassistant.nl/neuroradiology/dementia/role-of-mri
Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-98.
Zainal NH, Silva E, Lim LL, Kandiah N. Psychometric Properties of Alzheimer’s Disease Assessment Scale-Cognitive Subscale for Mild Cognitive Impairment and Mild Alzheimer’s Disease Patients in an Asian Context. Ann Acad Med Singap. 2016;45(7):273-83.
Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51(3):728-33.
Sotthibundhu A, Buntup D, Sanghirun C, Cherdchu K, Cheeramakara C, Chansirikarnjan S, et al. Low Serum Vitamin B12 in Alzheimers Patients as Detected by a Solid Phase Radioimmunoassay. Siriraj Med J. 2008;60(1):66-68.
Muangpaisan W. Rapidly Progressive Dementia due to Carcinomatous Meningitis Associated with Gastric Cancer. Siriraj Med J. 2016;68(1):47-50.
Suesat H, Srinonprasert V, Limpawattana P, Nakyos S, Poontananggul J, Jiraphorncharas C, et al. Detection of Postoperative Cognitive Dysfunction by Telemedicine Among Octogenarian Patients Who Underwent Minor Elective Surgery; Prospective Cohort Study. Siriraj Med J. 2022;74(2):126-33.
Piyapittayanan S, Segsarnviriya C, Ngamsombat C, Witthiwej T, Cheunsuchon P, Chawalparit O. Comparison between Dynamic Contrast-Enhanced MRI and Dynamic Susceptibility Contrast MRI in Glioma Grading. Siriraj Med J. 2017;69(6):369-76.
Yossie Susanti Eka Putri, Sitthimongkol Y, Wirojratana V, Chansatitporn N. Predictors of Depressive Symptoms among Family Caregivers of Patients with Dementia in Java, Indonesia. Siriraj Med J. 2021;73(8):549-58.
Piyapittayanan S, Segsarnviriya C, Ngamsombat C, Witthiwej T, Cheunsuchon P, Chawalparit O. Comparison between Dynamic Contrast-Enhanced MRI and Dynamic Susceptibility Contrast MRI in Glioma Grading. Siriraj Med J. 2017;69(6):369-76.
Ananwattanasuk J, Chiewvit P, Nilanont Y. Imaging Findings of CNS Lymphoma in Siriraj Hospital. Siriraj Med J. 2006;58(8):967-72.
Paganini-Hill A, Henderson VW. Estrogen replacement therapy and risk of Alzheimer’s disease. Arch Intern Med. 1996;156:2213-17.
Stephen CW, Randy JS, Denis GB, Michael WS. Insulin and the Blood-Brain Barrier. Curr Pharm Des. 2003;9:795-800.
Convit A. Links between cognitive impairment in insulin resistance: An explanatory model. Neurobiol Aging. 2005;26:31-35.
Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, et al. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage. 2011;54:2659-71.
Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J. Hypertens. 2008;26:1636-41.
Glodzik L, Mosconi L, Tsui W, de Santi S, Zinkowski R, Pirraglia E, et al. Alzheimer’s disease markers, hypertension, and gray matter damage in normal elderly. Neurobiol Aging. 2012;33:1215-27.
Gianaros PJ, Greer PJ, Ryan CM, Jennings JR. Higher blood pressure predicts lower regional grey matter volume: Consequences on short-term information processing. Neuroimage. 2006;31:754-65.
Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O’Brien JT, Ford GA. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Brain atrophy, WMH change and blood pressure. J Neurol. 2007;254:713-21.
Jennings JR, Mendelson DN, Muldoon MF, Ryan CM, Gianaros PJ, Raz N, et al. Regional grey matter shrinks in hypertensive individuals despite successful lowering of blood pressure. J Hum Hypertens. 2012;26:295-305.
Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Neurobiol. Aging. 2000;21:57-62.
Ashby EL, Miners JS, Kehoe PG, Love S. Effects of Hypertension and Anti-Hypertensive Treatment on Amyloid-ß Plaque Load and Aß-Synthesizing and Aß-Degrading Enzymes in Frontal Cortex. J Alzheimers Dis. 2016;50:1191-203.
Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328-35.
Sengupta P, Benjamin AI, Singh Y, Grover A. Prevalence and correlates of cognitive impairment in a north Indian elderly population. WHO South East Asia J Public Health. 2014;3(2):135-43.
Verdelho A, Madureira S, Moleiro C, Ferro JM, Santos CO, Erkinjuntti T, et al. White matter changes and diabetes predict cognitive decline in the elderly: the LADIS study. Neurology. 2010;75:160-7.
Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666.
Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426-36.
Van der Flier WM, van Straaten EC, Barkhof F, Verdelho A, Madureira S, Pantoni L, et al. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke. 2005;36:2116-20.
Verdelho A, Madureira S, Ferro JM, Basile AM, Chabriat H, Erkinjuntti T, et al. Differential impact of cerebral white matter changes, diabetes, hypertension and stroke on cognitive performance among non-disabled elderly. The LADIS study. J Neurol Neurosurg Psychiatry. 2007;78:1325-30.
Cavallin L, Bronge L, Zhang Y, Oksengard, AR, Wahlund LO, Fratiglioni L, et al. Comparison between visual assessment of MTA and hippocampal volumes in an elderly, non-demented population. Acta Radiol. 1987;53:573-9.
Westman E, Cavallin L, Muehlboeck J-S, Zhang Y, Mecocci P, Vellas B, et al. Sensitivity and specificity of medial temporal lobe visual ratings and multivar- iate regional MRI classification in Alzheimer’s disease. PLoS One. 2011;6:e22506.
Claus JJ, Staekenborg SS, Holl DC, Roorda JJ, Schuur J, Koster P, et al. Practical use of visual medial temporal lobe atrophy cut-off scores in Alzheimer’s disease: Validation in a large memory clinic population. Eur Radiol. 2017;27(8):3147-55.
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














