Consideration of Accuracy and Observational Error Analysis in Pelvic Sex Assessment: A Study in a Thai Cadaveric Human Population
DOI:
https://doi.org/10.33192/Smj.2022.40Keywords:
Morphometric analysis, forensic anthropology, sex estimation, technical error of measurementAbstract
Objective: In situations where skeletal human remains are recovered, pelvic bone morphology has been demonstrated to have an essential role in forensic sex identification. Determination of sex is one of the four pillars used to construct a biological profile of unidentified skeletal remains. Such analysis has mainly been confined to direct visual inspection or morphometric analysis of pelvic elements available. This study evaluates the identification accuracy and classification error established based on a morphometric sex determination of this bone either by direct observation or digital image analysis.
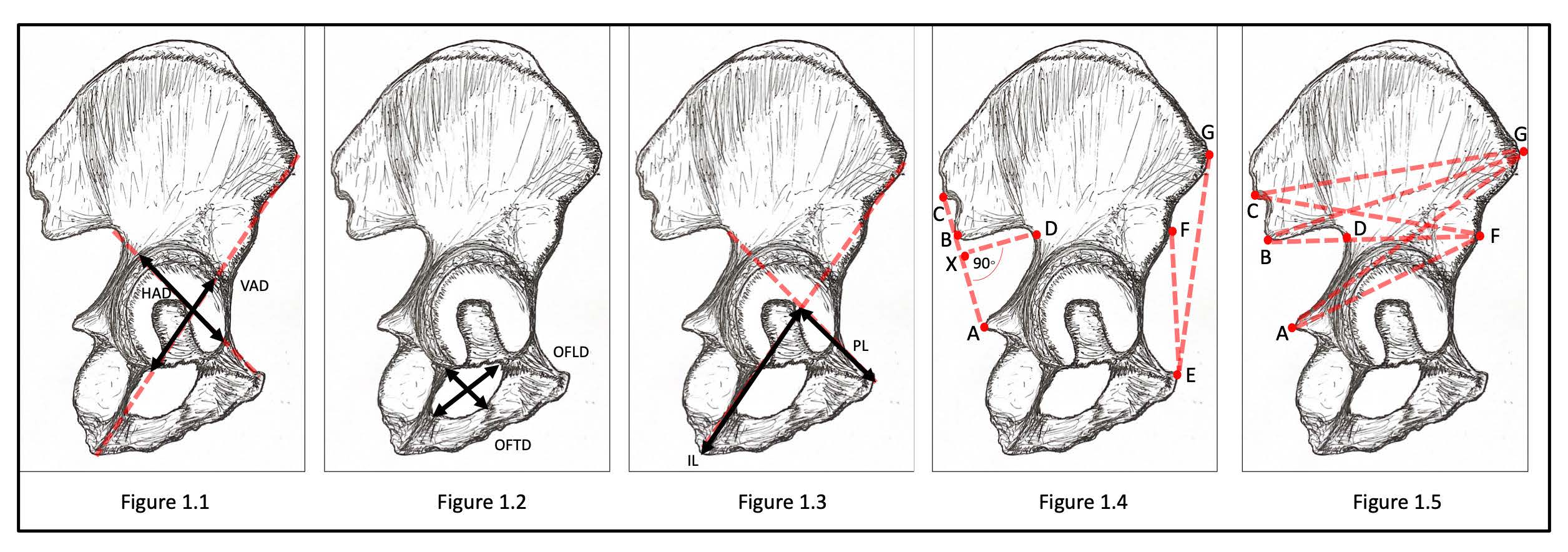
Materials and Methods: We used morphometric analysis of human pelvic bone from modern Thai samples to clarify the effect of variation in pelvic morphometric parameters on prediction accuracy. A total number of 408 pelvic bones (Male, n=249 and Female, n=159) were examined. Pelvic morphometric variables were measured in multiple regions for each bone.
Results: We found statistically significant differences in the pelvic morphometric parameters measured between the two sexes with considerably accurate classification and unavoidable errors by all means of analytical assessment.
Conclusion: Our findings suggest that it is not only variation of pelvic morphometric parameters between the two sexes in this population, but also the selection of analytical approach that can impact prediction accuracy and thus may contribute to the effect on the determination of sex. Ethical approval was not required for this study.
References
Christensen AM, Crowder CM. Evidentiary standards for forensic anthropology. J Forensic Sci. 2009;54(6):1211-6.
Christensen AM, Passalacqua NV. Introduction to Forensic Anthropology. In: A Laboratory Manual for Forensic Anthropology. London: Academic Press, 2018.
Christensen AM, Passalacqua NV, Bartelink EJ. Forensic Anthropology: Current Methods and Practice. London: Academic Press, 2014.
Krishan K, Chatterjee PM, Kanchan T, Kaur S, Baryah N, Singh RK. A review of sex estimation techniques during examination of skeletal remains in forensic anthropology casework. Forensic Sci Int. 2016;261:165.e1-8.
Kirkham GR, Cartmell SH. Genes and Proteins Involved in the Regulation of Osteogenesis. Genes Osteogenes. 2007;3(0):1-22.
Durić M, Rakočević Z, Donić D. The reliability of sex determination of skeletons from forensic context in the Balkans. Forensic Sci Int. 2005;147(2-3):159-64.
White T, Folkens P. The Human Bone Manual. Oxford: Academic, 2005.
Spradley MK, Jantz RL. Sex estimation in forensic anthropology: skull versus postcranial elements. J Forensic Sci. 2011;56(2):289-96.
Guyomarc’h P, Bruzek J. Accuracy and reliability in sex determination from skulls: A comparison of Fordisc® 3.0 and the discriminant function analysis. Forensic Sci Int. 2011;208(1-3):180.e1-6.
Bruzek J. A method for visual determination of sex, using the human hip bone. Am J Phys Anthropol. 2002;117(2):157-68.
Sangvichien S, Boonkaew K, Chuncharunee A. Sex Determination in Thai Skulls by Using Craniometry: Multiple Logistic Regression Analysis. Siriraj Med J. 2007;59:216-21.
Austin D, King RE. The Biological Profile of Unidentified Human Remains in a Forensic Context. Acad Forensic Pathol. 2016;6(3):370-90.
Rösing FW, Graw M, Marré B, Ritz-Timme S, Rothschild MA, Rötzscher K, et al. Recommendations for the forensic diagnosis of sex and age from skeletons. Homo. 2007;58(1):75-89.
González PN, Bernal V, Ivan Perez S, Barrientos G. Analysis of dimorphic structures of the human pelvis: its implications for sex estimation in samples without reference collections. J Archaeol Sci. 2007;34(10):1720-30.
Lesciotto KM, Doershuk LJ. Accuracy and Reliability of the Klales et al. (2012) Morphoscopic Pelvic Sexing Method. J Forensic Sci. 2018;63(1):214-20.
Phenice TW. A newly developed visual method of sexing the os pubis. Am J Phys Anthropol. 1969;30(2):297-301.
Ali SHM, Omar N, Shafie MS, Ismail NAN, Hadi H, Nor FM. Sex estimation using subpubic angle from reconstructed threedimensional computed tomography pelvic model in a contemporary Malaysian population. Anat Cell Biol. 2020;53(1):27-35.
Mahakkanukrauh P, Ruengdit S, Tun SM, Case DT, Sinthubua A. Osteometric sex estimation from the os coxa in a Thai population. Forensic Sci Int. 2017;271:127.e1-127.e7.
Bytheway JA, Ross AH. A geometric morphometric approach to sex determination of the human adult os coxa. J Forensic Sci. 2010;55(4):859-864.
Gonzalez PN, Bernal V, Perez SI. Geometric morphometric approach to sex estimation of human pelvis. Forensic Sci Int. 2009;189(1–3):68-74.
Chanapa P, Kijkuokool P, Singsuwan P, Mahakkanukrauh P. The most accurate sex determination using three morphologies in the pubis. Int Med J. 2018;25(3):189-192.
Rmoutilová R, Dupej J, Velemínská J, Brůžek J. Geometric morphometric and traditional methods for sex assessment using the posterior ilium. Leg Med. 2017;26:52-61.
Thieme F, Schull W. Sex determination from the skeleton. Hum Biol an Int Rec Res. 1957;29(3):242-73.
Drew R. A review of the ischium-pubis index: Accuracy, reliability, and common errors. Hum Biol. 2013;85(4):579-96.
Patriquin ML, Loth SR, Steyn M. Sexually dimorphic pelvic morphology in South African whites and blacks. HOMO- J Comp Hum Biol. 2003;53(3):255-62.
Patriquin ML, Steyn M, Loth SR. Metric analysis of sex differences in South African black and white pelves. Forensic Sci Int. 2005;147(2-3 SPEC.ISS.):119-27.
Sierp I, Henneberg M. The Difficulty of Sexing Skeletons from Unknown Populations. J Anthropol. 2015;2015:1-13.
Perini TA, de Oliveira GL, dos Santos Ornellas J, Palha de Oliveira F. Technical error of measurement in anthropometry. Rev Bras Med do Esporte. 2005;11(1):81-90.
Purkait R. Anthropometric landmarks: How reliable are they? Anthropometric landmarks. Medico-Legal Updat. 2004;4(4):133-40.
Ulijaszek SJ, Kerr DA. Anthropometric measurement error and the assessment of nutritional status. Br J Nutr. 1999;82(3):165-77.
Goto R, Mascie-Taylor CGN. Precision of measurement as a component of human variation. J Physiol Anthropol. 2007;26(2):253-6.
Jeyashree T, Sangeetha S, Premavathy D. Quantitative and qualitative morphometry of hip bone for determining sex. Drug Invent Today. 2019;11(10):2590-2. 33. Ubelaker DH, Volk CG. A Test of the Phenice Method for the Estimation of Sex. J Forensic Sci. 2002;47(1):19-24.
Kenyhercz MW, Klales AR, Stull KE, McCormick KA, Cole SJ. Worldwide population variation in pelvic sexual dimorphism: A validation and recalibration of the Klales et al. method. Forensic Sci Int. 2017;277:259.e1-259.e8.
Antony M, Mohanraj KG. Sex determination using geometric dimensions of greater sciatic notch and subpubic angle of human pelvic bone: A morphometric study. Drug Invent Today. 2019;12(10):2199-202.
Oikonomopoulou EK, Valakos E, Nikita E. Population-specificity of sexual dimorphism in cranial and pelvic traits: evaluation of existing and proposal of new functions for sex assessment in a Greek assemblage. Int J Legal Med. 2017;131(6):1731-8.
James MacAluso P. Sex determination from the acetabulum: Test of a possible non-population-specific discriminant function equation. J Forensic Leg Med. 2010;17(6):348-51.
Mary DJ, Mohanraj KG. Morphometric analysis of acetabulum and pubis of pelvic bone using acetabulopubic index. Drug Invent Today. 2019;12(10):2206-8.
Kanabur V. Identification of the sex of human hip bone by metric analysis of its anterior border. Biomed Res. 2012;23(2):211-214.
Brůžek J, Santos F, Dutailly B, Murail P, Cunha E. Validation and reliability of the sex estimation of the human os coxae using freely available DSP2 software for bioarchaeology and forensic anthropology. Am J Phys Anthropol. 2017;164(2):440-449.
Akshaya K, Mohanraj KG. Sex determination using the distance between posterior inferior iliac spine and ischial spine in dry human innominate bone. Drug Invent Today. 2019;11(9):2246-2249.
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














