Histopathological and Clinical Features of Methotrexate-Associated Lymphoproliferative Disorders and Post-Transplant Lymphoproliferative Disorders
DOI:
https://doi.org/10.33192/Smj.2022.69Keywords:
Methotrexate-associated lymphoproliferative disorders, post-transplant lymphoproliferative disorders, other iatrogenic immunodeficiency-associated lymphoproliferative disordersAbstract
Objective: To study histopathological and clinical features of methotrexate-associated lymphoproliferative disorders (MTX-LPD) and post-transplant lymphoproliferative disorders (PTLD).
Material and Methods: A retrospective study on 30 cases of MTX-LPD and 2 cases of PTLD from 2006 to 2021.
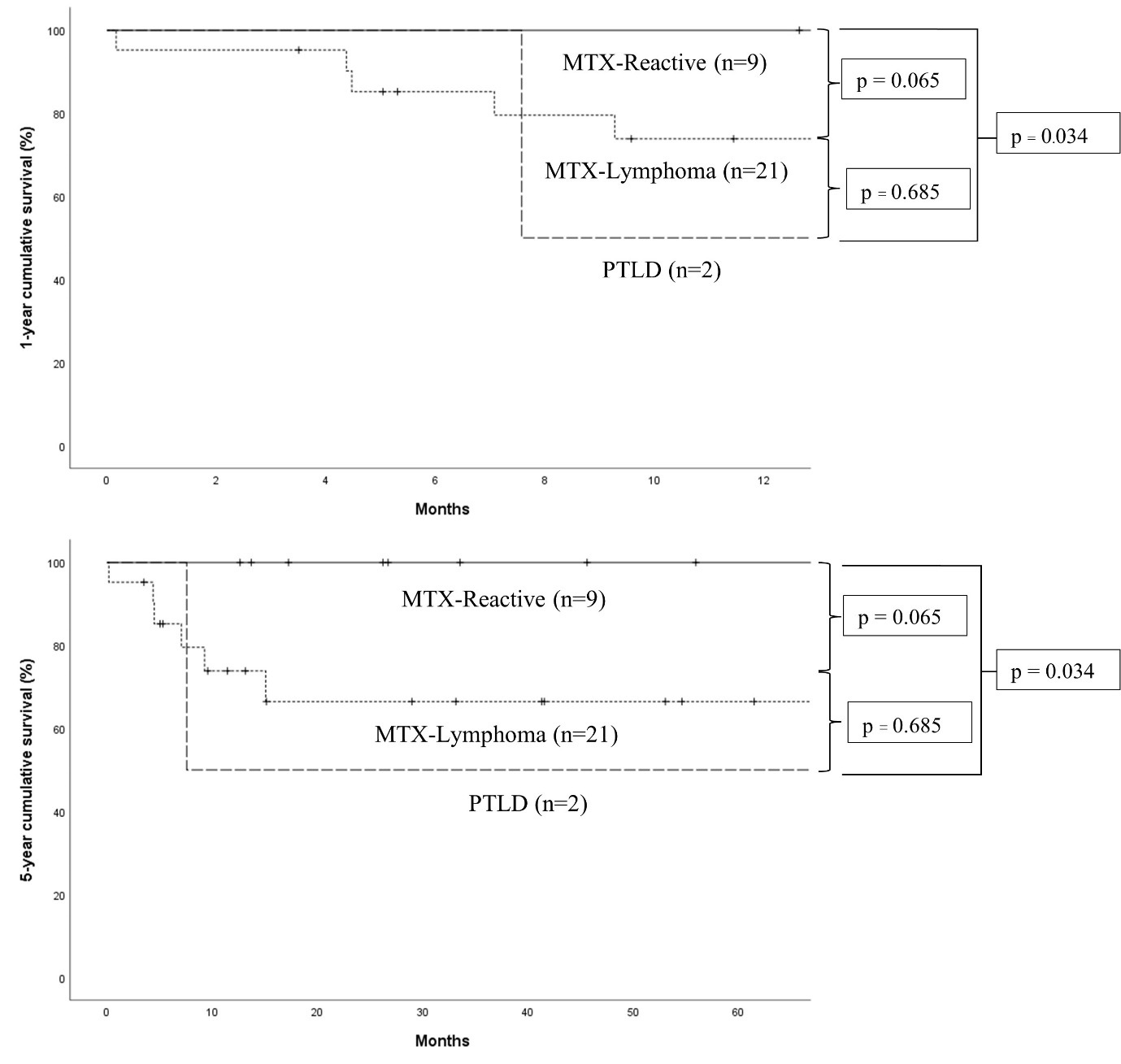
Results: By histopathology, the MTX-LPD group had 21 cases of lymphoma (MTX-Lymphoma) and 9 cases of reactive changes (MTX-Reactive). The PTLD group included diffuse large B-cell lymphoma and polymorphic PTLD (1 case each). The distinctive findings in MTX-Lymphoma and PTLD were association with Epstein-Barr virus (EBV) (8/12 cases, 66.7%) and CD30 positivity (13/18 cases, 72.2%). The MTX-LPD group had median MTX dosage of 10 mg/week, median MTX cumulative dosage of 2,613.75 mg, and median duration of MTX usage of 2,186 days. The 14 MTX-LPD and PTLD patients has median duration to response after the varied interventions of 47 days and the time to the first complete remission (CR) of 126 days. The MTX-Reactive patients had a significantly higher absolute lymphocyte count, younger median age, fewer B symptoms, higher rate of single site involvement, less extranodal involvement, shorter duration to response, less time to enter CR, and higher CR rate than the MTXLymphoma patients (p < 0.05).
Conclusion: Histopathology in MTX-LPD and PTLD patients can vary from reactive changes to lymphoma. EBV study and CD30 immunostaining help identify MTX-LPD and PTLD. History of MTX usage and other causes of immunodeficiency should be considered before diagnosing lymphoma. MTX discontinuation or reduction of immunosuppressant dosage are recommended before administrating combined chemotherapeutic agents in unresponsive cases.
References
Koźmiński P, Halik PK, Chesori R, Gniazdowska E. Overview of dual-acting drug methotrexate in different neurological diseases, autoimmune pathologies and cancers. Int J Mol Sci. 2020;21:3483.
Salloum E, Cooper DL, Howe G, Lacy J, Tallini G, Crouch J, et al. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol. 1996;14:1943-49.
Mariette X, Cazals-Hatem D, Warszawki J, Liote F, Balandraud N, Sibilia J, et al. Lymphomas in rheumatoid arthritis patients treated with methotrexate: a 3-year prospective study in France. Blood. 2002;99:3909-15.
Hoshida Y, Xu J-X, Fujita S, Nakamichi I, Ikeda J-I, Tomita Y, et al. Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol. 2007;34:322-31.
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues (Revised 4th edition). Lyon: IARC; 2017.
Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36:1720-48.
Inui Y, Matsuoka H, Yakushijin K, Okamura A, Shimada T, Yano S, et al. Methotrexate-associated lymphoproliferative disorders: management by watchful waiting and observation of early lymphocyte recovery after methotrexate withdrawal. Leuk Lymphoma. 2015;56:3045-51.
Kitamura N, Sugiyama K, Nagasawa Y, Hamaguchi M, Kobayashi H, Takei M. Involvement of Epstein-Barr virus in the development and spontaneous regression of methotrexate-associated lymphoproliferative disorder in patients with rheumatoid arthritis. Clin Exp Rheumatol 2022;40:1330-5.
Gion Y, Doi M, Nishimura Y, Ikeda T, Filiz Nishimura M, Sakamoto M, et al. PD-L1 expression is associated with the spontaneous regression of patients with methotrexate-associated lymphoproliferative disorders. Cancer Med 2022;11:417-32.
Harada T, Iwasaki H, Muta T, Urata S, Sakamoto A, Kohno K, et al. Outcomes of methotrexate-associated lymphoproliferative disorders in rheumatoid arthritis patients treated with diseasemodifying anti-rheumatic drugs. Br J Haematol 2021;194:101-10.
Fransen J, Stucki G, van Riel PLCM. Rheumatoid arthritis measures: Disease Activity Score (DAS), Disease Activity Score-28 (DAS28), Rapid Assessment of Disease Activity in Rheumatology (RADAR), and Rheumatoid Arthritis Disease Activity Index (RADAI). Arthritis Care & Rheumatism 2003;49:S214-24.
Kurita D, Miyoshi H, Ichikawa A, Kato K, Imaizumi Y, Seki R, et al. Methotrexate-associated lymphoproliferative disorders in patients with rheumatoid arthritis: clinicopathologic features and prognostic factors. Am J Surg Pathol. 2019 Jul;43:869-84.
Ichikawa A, Arakawa F, Kiyasu J, Sato K, Miyoshi H, Niino D, et al. Methotrexate/iatrogenic lymphoproliferative disorders in rheumatoid arthritis: histology, Epstein-Barr virus, and clonality are important predictors of disease progression and regression. Eur J Haematol. 2013;91:20-8.
Yamakawa N, Fujimoto M, Kawabata D, Terao C, Nishikori M, Nakashima R, et al. A clinical, pathological, and genetic characterization of methotrexate-associated lymphoproliferative disorders. J Rheumatol. 2014;41:293-9.
Tokuhira M, Saito S, Okuyama A, Suzuki K, Higashi M, Momose S, et al. Clinicopathologic investigation of methotrexateinduced lymphoproliferative disorders, with a focus on regression. Leuk Lymphoma. 2018;59:1143-52.
Nakano K, Saito K, Nawata A, Hanami K, Kubo S, Miyagawa I, et al. Clinical aspects in patients with rheumatoid arthritis complicated with lymphoproliferative disorders without regression after methotrexate withdrawal and treatment for arthritis after regression of lymphoproliferative disorders. Mod Rheumatol. 2021;31:94-100.
Satou A, Tabata T, Miyoshi H, Kohno K, Suzuki Y, Yamashita D, et al. Methotrexate-associated lymphoproliferative disorders of T-cell phenotype: clinicopathological analysis of 28 cases. Mod Pathol. 2019;32:1135-46.
Yamada K, Oshiro Y, Okamura S, Fujisaki T, Kondo S, Nakayama Y, et al. Clinicopathological characteristics and rituximab addition to cytotoxic therapies in patients with rheumatoid arthritis and methotrexate-associated large B lymphoproliferative disorders. Histopathology. 2015;67:70-80.
Koens L, Senff NJ. Vermeer MH, Willemze R, Jansen PM. Methotrexate-associated B-cell lymphoproliferative disorders presenting in the skin: A clinicopathologic and immunophenotypical study of 10 cases Am J Surg Pathol. 2014;38:999-1006.
Tokuhira M, Tamaru J-I, Kizaki M. Clinical management for other iatrogenic immunodeficiency-associated lymphoproliferative disorders. J Clin Exp Hematop. 2019;59:72-92.
Dierickx D, Vergote V. Management of Post-transplant Lymphoproliferative Disorders. Hema Sphere, 2019;3:74-7.
Saito S, Kaneko Y, Yamaoka K, Tokuhira M, Takeuchi T. Distinct patterns of lymphocyte count transition in lymphoproliferative disorder in patients with rheumatoid arthritis treated
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














