Untargeted Metabolomics Analysis using LC-MSQTOF for Metabolite Profile Comparison between Patients with Myofascial Pain of Upper Trapezius Muscle versus Controls
DOI:
https://doi.org/10.33192/Smj.2022.94Keywords:
Myofascial pain, trigger point, metabolomics, untargeted metabolomics, mass spectrometryAbstract
Objective: This study aims to identify different biomarkers of Myofascial pain syndrome (MPS) using untargeted metabolomics screening.
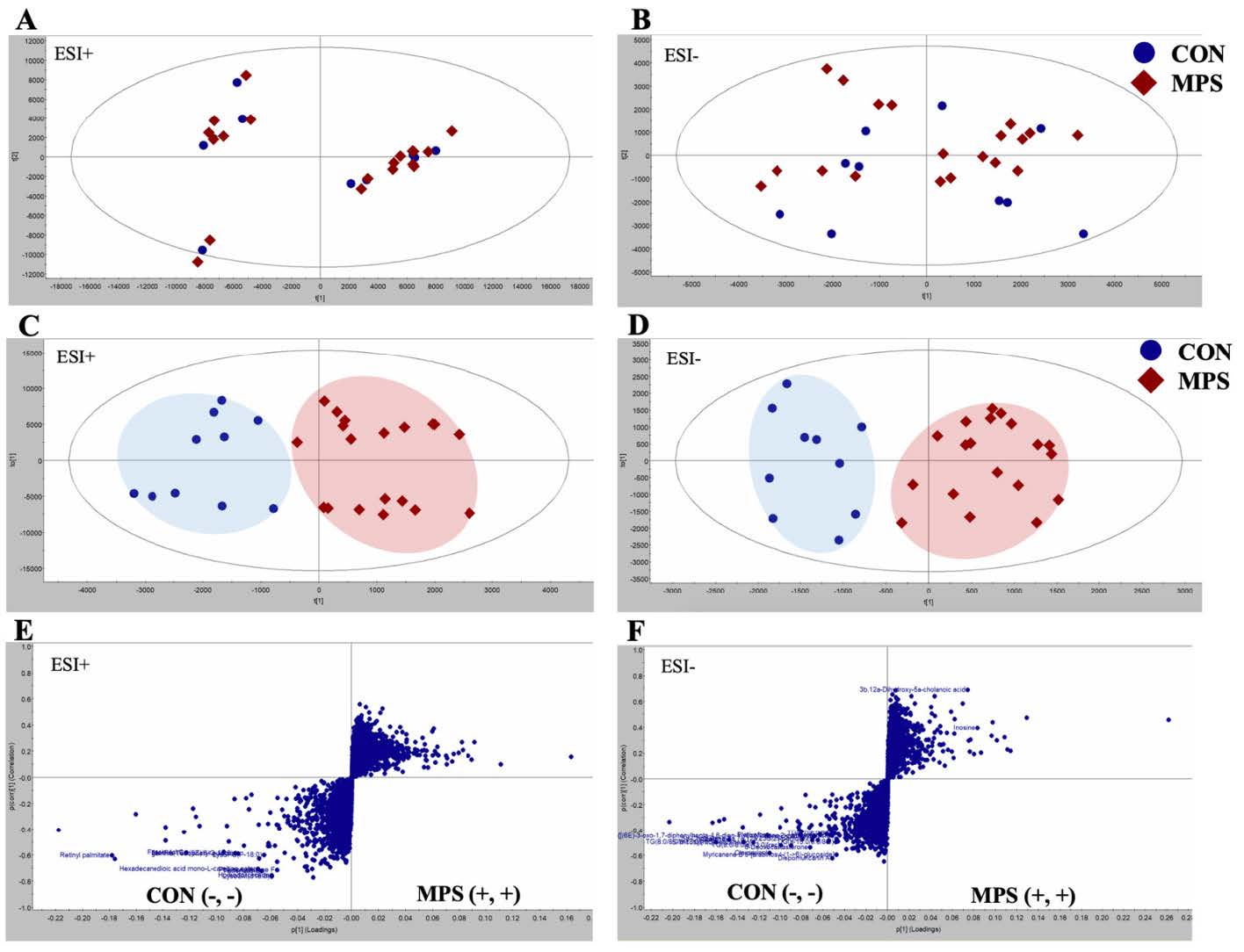
Materials and Methods: In a case-control study, serum samples from MPS patients (n = 19) and healthy controls (n = 10) were analyzed using reverse-phase liquid chromatography and mass spectrometry quadrupole time-of-flight (MS-QTOF). The resulted raw data was processed with Progenesis QI data analysis software. The HMBD database was used to identify the metabolites based on their fold change (>1.2), variable importance plot (>1) with P < 0.05. MetaboAnalyst 5.0 was used to generate metabolic network analysis for all identified metabolites.
Results: The MPS group reported significantly higher pain on visual analog scale when compared with control while most of the other routine blood chemical profiles were not different. Twenty-seven metabolites were analyzed and identified with untargeted metabolomics analysis which could distinguish MPS patients from healthy controls. Inosine and chenodeoxycholic acid were abundant in the MPS group, whereas the others were low. Metabolites were divided into three categories: lipids, nucleotides, and organic compounds. Possible MPS metabolites included lysoSM (sphingomyelin), lysoPC (lysophosphatidylcholine), lysoPE (lysophosphatidylethanolamine), triglyceride, and inosine.
Conclusion: These metabolite profiles, including glycerophospholipids mechanism and purine metabolism, indicate that the inflammatory process might be related to the mechanisms of MPS. A larger sample size, a different trigger point location, and modifications in therapy afterward should all be further explored.
References
Fejer R, Kyvik K, Hartvigsen J. The prevalence of neck pain in the world population: a systematic critical review of the literature. Eur Spine J. 2006;15:834-48.
Fernández-de-las-Peñas C, Dommerholt J. International Consensus on Diagnostic Criteria and Clinical Considerations of Myofascial Trigger Points: A Delphi Study. Pain Medicine. 2017;19:142-50.
Borg-Stein J, Simons D. Myofascial pain. Arch Phys Med Rehabil. 2002;83:S40- S7.
Huguenin L. Myofascial trigger points: the current evidence. Physical Therapy in Sport. 2004;5:2-12.
Cerezo-Tellez E, Torres-Lacomba M, Mayoral-Del Moral O, Sanchez-Sanchez B, Dommerholt J, Gutierrez-Ortega C. Prevalence of Myofascial Pain Syndrome in Chronic Non Specific Neck Pain: A Population-Based Cross-Sectional Descriptive Study. Pain Med. 2016;17:2369-77.
Jafri M. Mechanisms of myofascial pain. Int Sch Res Notices. 2014.
Mense S. Morphology of myofascial trigger points: What does a trigger point look like? Springer. 86-100.
Adigozali H, Shadmehr A, Ebrahimi E, Rezasoltani A, Naderi F. Reliability of upper trapezius morphology, its mechanical properties and blood flow in female patients with myofascial pain syndrome using ultrasonography. J Bodyw Mov Ther. 2017;21(1):35-40.
Hong C-Z, Simons D. Pathophysiologic and electrophysiologic mechanisms of myofascial trigger points. Arch Phys Med Rehabil. 1998;79:863-72.
Pedersen-Bjergaard U, Nielsen L, Jensen K, Edvinsson L, Jansen I, Olesen J. Calcitonin gene-related peptide, neurokinin A and substance P: effects on nociception and neurogenic inflammation in human skin and temporal muscle. Peptides. 1991;12:333-7.
Shah J, Gilliams E. Uncovering the biochemical milieu of myofascial trigger points using in vivo microdialysis: an application of muscle pain concepts to myofascial pain syndrome. J Bodyw Mov Ther. 2008;12:371-84.
Shah J, Phillips T, Danoff J, Gerber L. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol (1985). 2005;99:1977-84.
Nielsen J, Oliver S. The next wave in metabolome analysis. Trends in biotechnology. 2005;23:544-6.
Klupczynska A, Derezinski P, Korot Z. Metabolomics in medical sciences - trends, challenges and perspectives. Acta Pol Pharm. 2015;72:629-41.
Holmes E, Wilson I, Nicholson J. Metabolic phenotyping in health and disease. Cell. 2008;134:714-7.
Hadrévi J, Björklund M, Kosek E, Hällgren S, Antti H, Fahlström M, et al. Systemic differences in serum metabolome: a cross sectional comparison of women with localised and widespread pain and controls. Scientific Reports. 2015;5:15925.
Dunn WB, Broadhurst D, Begley P, Zelena E, Francis-McIntyre S, Anderson N, et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nature Protocols. 2011;6:1060-83.
Vazquez-Delgado E, Cascos-Romero J, Gay-Escoda C. Myofascial pain syndrome associated with trigger points: a literature review. (I): Epidemiology, clinical treatment and etiopathogeny. Med Oral Patol Oral Cir Bucal. 2009;14:e494-8.
Farooqui AA, Horrocks LA, Farooqui T. Glycerophospholipids in brain: their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem Phys Lipids. 2000;106:1-29.
Han X. Lipidomics for studying metabolism. Nat Rev Endocrinol. 2016;12:668-79.
Berens RL, Krug EC, Marr JJ. 6 - Purine and pyrimidine metabolism. In: Marr JJ, Müller M, editors. Biochemistry and Molecular Biology of Parasites. San Diego: Academic Press; 1995.p.89-117.
Jinnah HA, Sabina Rl, Van Den Berghe G. Metabolic disorders of purine metabolism affecting the nervous system. In: Dulac O, Lassonde M, Sarnat HB, editors. Handbook of Clinical Neurology. 113: Elsevier; 2013.p.1827-36.
Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58-67.
Nixon GF. Sphingolipids in inflammation: pathological implications and potential therapeutic targets. Br J Pharmacol. 2009;158:982-93.
Patti GJ, Yanes O, Siuzdak G. Metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol. 2012;13:263-9.
Peterlin BL, Mielke MM, Dickens AM, Chatterjee S, Dash P, Alexander G, et al. Interictal, circulating sphingolipids in women with episodic migraine: A case-control study. Neurology. 2015;85:1214-23.
Ale A, Argyriou AA, Bruna J. Sphingolipid metabolism products: potential new players in the pathogenesis of bortezomibinduced neuropathic pain. Ann Transl Med. 2018;6:S78.
Finco G, Locci E, Mura P, Massa R, Noto A, Musu M, et al. Can urine metabolomics be helpful in differentiating neuropathic and nociceptive pain? a proof-of-concept study. PLoS One. 2016;11:e0150476.
Tokumura A, Nishioka Y, Yoshimoto O, Shinomiya J, Fukuzawa K. Substrate specificity of lysophospholipase D which produces bioactive lysophosphatidic acids in rat plasma. Biochim Biophys Acta. 1999;1437:235-45.
Dinkla S, van Eijk LT, Fuchs B, Schiller J, Joosten I, Brock R, et al. Inflammation-associated changes in lipid composition and the organization of the erythrocyte membrane. BBA Clin. 2016;5:186-92.
Zhai G, Pelletier JP, Liu M, Randell EW, Rahman P, Martel-Pelletier J. Serum lysophosphatidylcholines to phosphatidylcholines ratio is associated with symptomatic responders to symptomatic drugs in knee osteoarthritis patients. Arthritis Res Ther. 2019;21:224.
Menzies V, Starkweather A, Yao Y, Thacker LR, 2nd, Garrett TJ, Swift-Scanlan T, et al. Metabolomic differentials in women with and without fibromyalgia. Clin Transl Sci. 2020;13:67-77.
Caboni P, Liori B, Kumar A, Santoru ML, Asthana S, Pieroni E, et al. Metabolomics analysis and modeling suggest a lysophosphocholines-PAF receptor interaction in fibromyalgia. PLoS One. 2014;9:e107626.
Ren C, Liu J, Zhou J, Liang H, Wang Y, Sun Y, et al. Lipidomic analysis of serum samples from migraine patients. Lipids Health Dis. 2018;17:22.
Kuwajima K, Sumitani M, Kurano M, Kano K, Nishikawa M, Uranbileg B, et al. Lysophosphatidic acid is associated with neuropathic pain intensity in humans: An exploratory study. PLoS One. 2018;13:e0207310.
Rockel JS, Kapoor M. The metabolome and osteoarthritis: possible contributions to symptoms and pathology. Metabolites. 2018;8:92.
van der Kleij D, Yazdanbakhsh M. Control of inflammatory diseases by pathogens: lipids and the immune system. Eur J Immunol. 2003;33:2953-63.
Castoldi A, Monteiro LB, van Teijlingen Bakker N, Sanin DE, Rana N, Corrado M, et al. Triacylglycerol synthesis enhances macrophage inflammatory function. Nat Commun. 2020;11:4107.
Starkweather A, Julian T, Ramesh D, Heineman A, Sturgill J, Dorsey SG, et al. Circulating lipids and acute pain sensitization: an exploratory analysis. Nurs Res. 2017;66:454-61.
Lee J, Ridgway ND. Phosphatidylcholine synthesis regulates triglyceride storage and chylomicron secretion by Caco2 cells. J Lipid Res. 2018;59:1940-50.
Nascimento F, Jr Sr, Santos A. The Involvement of Purinergic System in Pain: Adenosine Receptors and Inosine as Pharmacological Tools in Future Treatments. 2012.
Modis K, Gero D, Stangl R, Rosero O, Szijarto A, Lotz G, et al. Adenosine and inosine exert cytoprotective effects in an in vitro model of liver ischemia-reperfusion injury. Int J Mol Med. 2013;31:437-46.
de Oliveira ED, Schallenberger C, Bohmer AE, Hansel G, Fagundes AC, Milman M, et al. Mechanisms involved in the antinociception induced by spinal administration of inosine or guanine in mice. Eur J Pharmacol. 2016;772:71-82.
Cinalli AR, Guarracino JF, Fernandez V, Roquel LI, Losavio AS. Inosine induces presynaptic inhibition of acetylcholine release by activation of A3 adenosine receptors at the mouse neuromuscular junction. Br J Pharmacol. 2013;169:1810-23.
Fais A, Cacace E, Corda M, Era B, Peri M, Utzeri S, et al. Purine metabolites in fibromyalgia syndrome. Clin Biochem. 2013;46:37-9.
Alexander GM, Reichenberger E, Peterlin BL, Perreault MJ, Grothusen JR, Schwartzman RJ. Plasma amino acids changes in complex regional pain syndrome. Pain Res Treat. 2013;2013:742407.
Clos-Garcia M, Andrés-Marin N, Fernández-Eulate G, Abecia L, Lavín JL, van Liempd S, et al. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. EBioMedicine. 2019;46:499-511.
Hackshaw KV, Rodriguez-Saona L, Plans M, Bell LN, Buffington CA. A bloodspot-based diagnostic test for fibromyalgia syndrome and related disorders. Analyst. 2013;138:4453-62.
Zhang W, Sun G, Likhodii S, Liu M, Aref-Eshghi E, Harper PE, et al. Metabolomic analysis of human plasma reveals that arginine is depleted in knee osteoarthritis patients. Osteoarthritis Cartilage. 2016;24:827-34.
Hadrévi J, Ghafouri B, Sjörs A, Antti H, Larsson B, Crenshaw AG, et al. Comparative metabolomics of muscle interstitium fluid in human trapezius myalgia: an in vivo microdialysis study. Eur J Appl Physiol. 2013;113:2977-89.
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














