Clinical Features of Adult Male Acne in a Tropical Country: A Prospective Cross-sectional Study
DOI:
https://doi.org/10.33192/smj.v75i2.260742Keywords:
Acne vulgaris, adult acne, maleAbstract
Objective: To evaluate the characteristics of post-adolescent male patients with acne in terms of the onset of the condition, its clinical course and severity, and the behaviors associated with its severity.
Materials and Methods: A prospective, cross-sectional study was conducted on adult males with acne who visited Siriraj Hospital, Thailand. All male acne patients aged 21 years and older were enrolled. Diagnoses and physical examinations were performed by dermatologists.
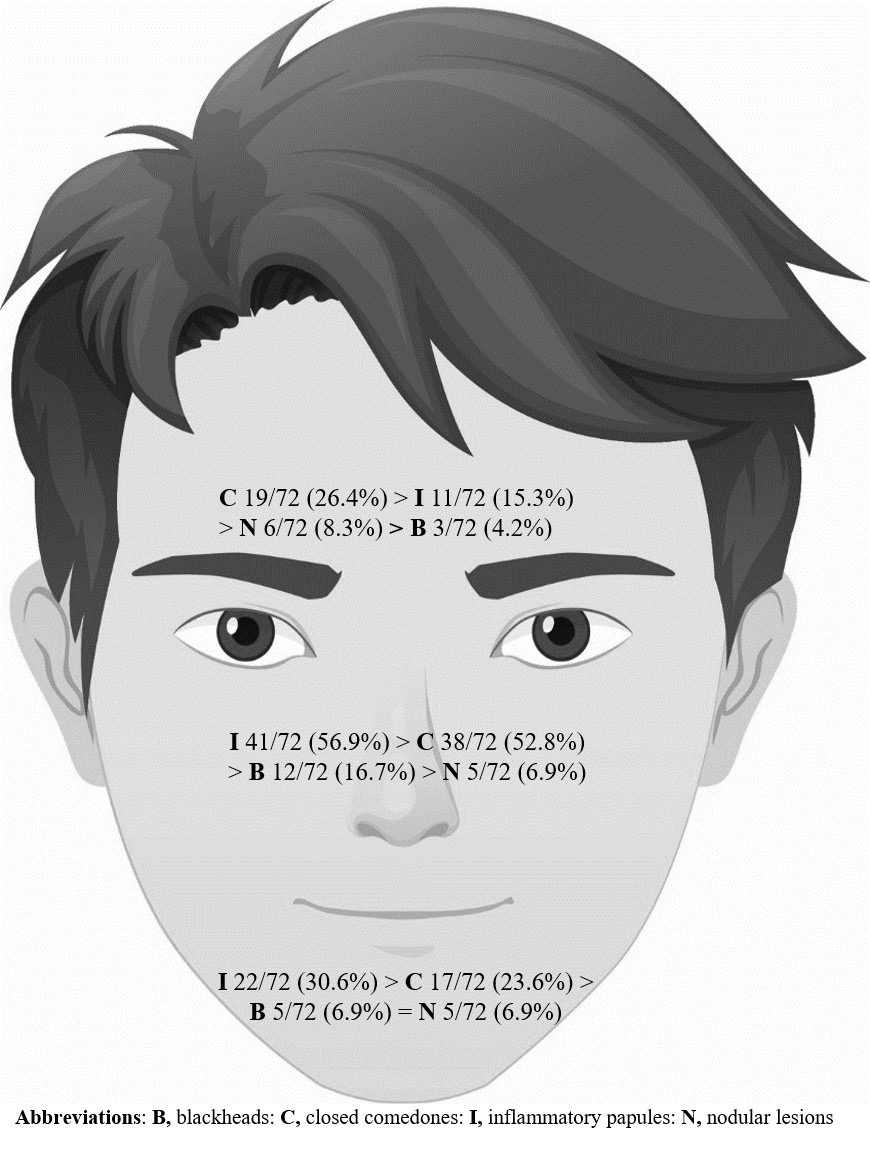
Results: Seventy-two patients (mean age, 26.9 [± 4.3] years) were included. Persistent acne, relapse acne, and late-onset acne (onset at age ≥ 21 years) were reported in 62.5%, 33.3%, and 4.2% cases, respectively. Persistent acne tended to subside at 26 years of age, whereas late-onset acne tended to start at 28 years of age. The acne severity was mild in most cases. Pimple-picking, followed by frequent face washing, were common habits among male acne patients. Shaving influenced the severity in some adult male with acne.
Conclusion: Adult male acne commonly presented as inflammatory lesions and comedones on the cheeks. They commonly had an onset earlier than 21 years old and continued into adulthood, but the post-adolescent severity tended to be mild. While several factors have been reported elsewhere to be involved in the severity of acne, this study found that only shaving influenced severity.
References
Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
Thiboutot DM, Dréno B, Abanmi A, Alexis AF, Araviiskaia E, Barona Cabal MI, et al. Practical management of acne for clinicians: An international consensus from the Global Alliance to Improve Outcome in Acne. J Am Acad Dermatol. 2018;78:S1-S23.e1.
Skroza N, Tolino E, Mambrin A, Zuber S, Balduzzi V, Marchesiello A, et al. Adult acne versus adolescent acne: a retrospective study of 1,167 patients. J Clin Aesthet Dermatol. 2018;11:21-5.
Chlebus E, Chlebus M. Factor affecting the course and severity of adult acne: Observational cohort study. J Dermatolog Treat. 2017;28:737-44.
Penso L, Touvier M, Deschasaux M, Szabo de Edelenyi F, Hercberg S, Ezzedine K, et al. Association between adult acne and dietary behaviors findings from the Nutrinet-Sante’ Prospective cohort study. JAMA Dermatol. 2020;156:854-62.
Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014; 27 Suppl 1:3-8.
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Acne Vulgaris: establishing effectiveness of drugs intended for treatment guidance for industry [Internet]. 2018 [cited 2021 Jan 21]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/acne-vulgaris-establishing-effectiveness-drugs-intended-treatment.
Clark AK, Saric S, Sivamani RK. Acne Scars: How Do We Grade Them? Am J Clin Dermatol. 2018;19:139-44.
Kaminsky A, Florez-White M, Bagatin E, Arias MI. Large prospective study on adult acne in Latin America and the Iberian Peninsula: risk factors, demographics, and clinical characteristics. Int J Dermatol. 2019;58:1277-82.
Bagatin E, Freitas THP, Rivitti-Machado MC, Machado MCR, Ribeiro BM, Nunes S, et al. Adult female acne: a guide to clinical practice. An Bras Dermatol. 2019;94:62-75.
Goulden V, McGeown H, Cunliffe WJ. The familial risk of adult acne: comparison between first-degree relatives of affected and unaffected individuals. Br J Dermatol. 1999;141:297-300.
Ismail NH, Manaf ZA, Azizan NZ. High glycemic load diet, milk and ice cream consumption are related to acne vulgaris in Malaysian young adults: a case control study. BMC Dermatol. 2012;12:13.
Schäfer T, Nienhaus A, Vieluf D, Berger J, Ring J. Epidemiology of acne in the general population: the risk of smoking. Br J Dermatol. 2001;145:100-4.
Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78:335-41.
Chlebus E, Chlebus M. The role of adolescent acne treatment in formation of scars Among patients with persistent adult acne: evidence from an observational study. Cutis. 2019;104:57-61.
Rawlings AV. Ethnic skin types: are there differences in skin structure and function? Int J Cosmet Sci. 2006;28:79-93.
Klaz I, Kochba I, Shohat T, Zarka S, Brenner S. Severe acne vulgaris and tobacco smoking in young men. J Invest Dermatol. 2006;126:1749-52.
Wei B, Pang Y, Zhu H, Qu L, Xiao T, Wei HC, et al. The epidemiology of adolescent acne in North East China. J Eur Acad Dermatol Venereol. 2010;24:953-7.
Karadağ AS, Balta I, Saricaoğlu H, Kiliç S, Kelekçi KH, Yildirim M, et al. The effect of personal, familial, and environmental characteristics on acne vulgaris: a prospective, multicenter, case controlled study. G Ital Dermatol Venereol. 2019;154:177-85.
Xu SX, Wang HL, Fan X, Sun LD, Yang S, Wang PG, et al. The familial risk of acne vulgaris in Chinese Han—a case-control study. J Eur Acad Dermatol Venereol. 2007;21:602-5.
Wolkenstein P, Misery L, Amici JM, Maghia R, Branchoux S, Cazeau C, et al. Smoking and dietary factors associated with moderate-to-severe acne in French adolescents and young adults: results of a survey using a representative sample. Dermatology. 2015;230:34-9.
Di Landro A, Cazzaniga S, Parazzini F, Ingordo V, Cusano F, Atzori L, et al. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults. J Am Acad Dermatol. 2012;67:1129-35.
Aalemi AK, Anwar I, Chen H. Dairy consumption and acne: a case control study in Kabul, Afghanistan. Clin Cosmet Investig Dermatol. 2019;12:481-7.
Wolkenstein P, Machovcová A, Szepietowski JC, Tennstedt D, Veraldi S, Delarue A. Acne prevalence and associations with lifestyle: a cross-sectional online survey of adolescents/young adults in 7 European countries. J Eur Acad Dermatol Venereol. 2018;32:298-306.
Ibrahim AA, Salem RM, El-Shimi OS, Baghdady SMA, Hussein S. IL1A (-889) gene polymorphism is associated with the effect of diet as a risk factor in acne vulgaris. J Cosmet Dermatol. 2019;18:333-6.
Published
How to Cite
Issue
Section
Categories
License

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














