Diphenylcyclopropenone Treatment Outcomes for Alopecia Areata
DOI:
https://doi.org/10.33192/smj.v75i2.260751Keywords:
Alopecia areata, Diphenylcyclopropenone, Diphencyprone, DCP, DPCP, Topical immunotherapy, PrognosisAbstract
Objective: To ascertain (1) Diphenylcyclopropenone (DCP)’s efficacy in treating alopecia areata (AA), alopecia totalis (AT), and alopecia universalis (AU) in Thai patients; and (2) prognostic factors.
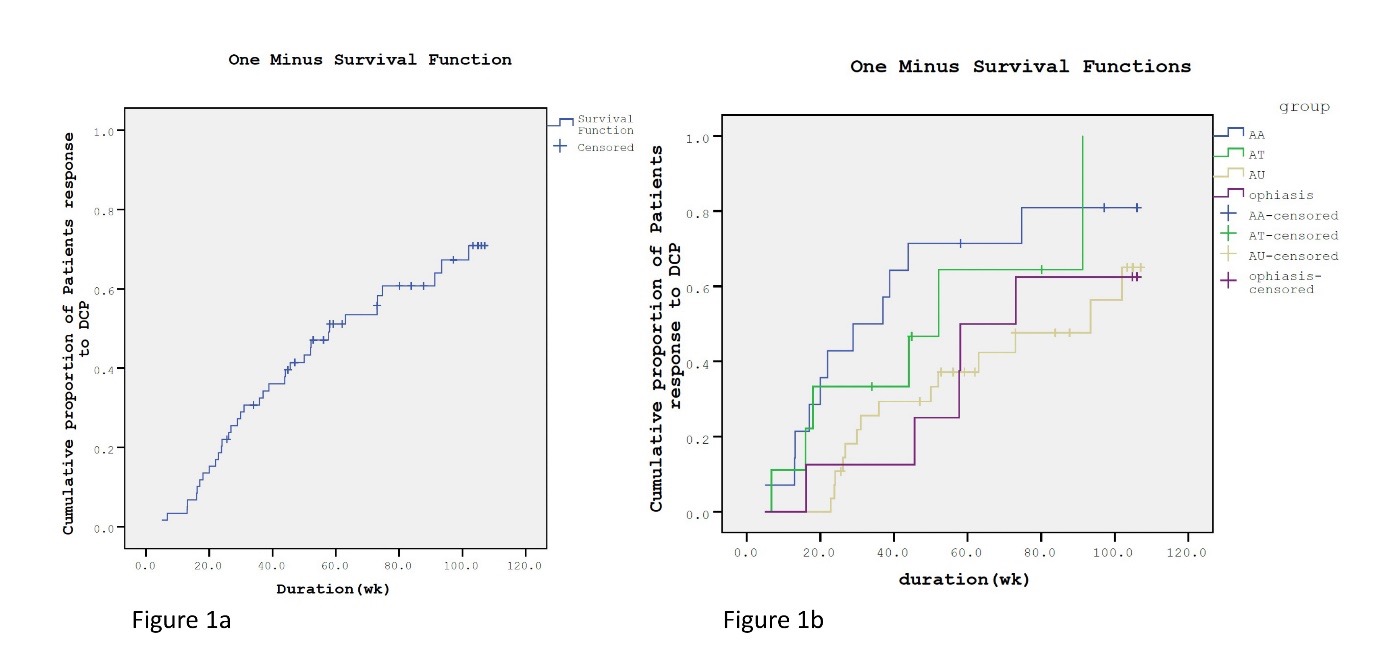
Materials & Methods: We retrospectively reviewed the medical records of patients with AA, AT, and AU who were administered DCP at Siriraj Hospital, Bangkok, Thailand. The median response and relapse times of the 3 groups were evaluated. Factors affecting outcomes were investigated.
Results: Fifty-nine cases were enrolled (AA, 22; AT, 9; AU, 28), with women predominating in each group. The overall response was 61% (AA, 78.6%; AT, 66.7%; AU, 50%). The median response time was 58 weeks, with a significantly longer time for AU than AA (P = 0.006). Factors significantly influencing response to DCP, evaluated by multivariate analysis, were older age at onset (P = 0.02), disease duration before DCP initiation (P = 0.003), and treatment duration to initial hair regrowth (P = 0.001). The overall relapse rate was 63.9%, with a median of 39 weeks between response and relapse. The most common side effect was blistering (73.7%).
Conclusion: DCP is effective and safe for treating extensive AA. Favorable prognostic factors are low disease severity, late disease onset, short duration before DCP treatment, and short duration to initial response. As the relapse rate is high, maintenance therapy should be considered.
References
Rokhsar CK, Shupack JL, Vafai JJ, Washenik K. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol 1998;39:751-61.
Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part II. Treatment. J Am Acad Dermatol 2010;62:191-202.
Happle R. Antigenic competition as a therapeutic concept for alopecia areata. Arch Dermatol Res 1980;267:109-14.
Herbst V, Zoller M, Kissling S, Wenzel E, Stutz N, Freyschmidt-Paul P. Diphenylcyclopropenone treatment of alopecia areata induces apoptosis of perifollicular lymphocytes. Eur J Dermatol 2006;16:537-42.
Happle R, Klein HM, Macher E. Topical immunotherapy changes the composition of the peribulbar infiltrate in alopecia areata. Arch Dermatol Res 1986;278:214-8.
Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part I. Clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol 2010;62:177-88.
Kyriakis KP, Paltatzidou K, Kosma E, Sofouri E, Tadros A, Rachioti E. Alopecia areata prevalence by gender and age. J Eur Acad Dermatol Venereol 2009;23:572-3.
Olsen EA, Hordinsky MK, Price VH, Roberts JL, Shapiro J, Canfield D, et al. Alopecia areata investigational assessment guidelines--Part II. National Alopecia Areata Foundation. J Am Acad Dermatol 2004;51:440-7.
El-Zawahry BM, Bassiouny DA, Khella A, Zaki NS. Five-year experience in the treatment of alopecia areata with DPC. J Eur Acad Dermatol Venereol 2010;24:264-9.
Hull SM, Norris JF. Diphencyprone in the treatment of long-standing alopecia areata. Br J Dermatol 1988;119:367-74.
Sotiriadis D, Patsatsi A, Lazaridou E, Kastanis A, Vakirlis E, Chrysomallis F. Topical immunotherapy with diphenylcyclopropenone in the treatment of chronic extensive alopecia areata. Clin Exp Dermatol 2007;32:48-51.
Avgerinou G, Gregoriou S, Rigopoulos D, Stratigos A, Kalogeromitros D, Katsambas A. Alopecia areata: topical immunotherapy treatment with diphencyprone. J Eur Acad Dermatol Venereol 2008;22:320-3.
Wiseman MC, Shapiro J, MacDonald N, Lui H. Predictive model for immunotherapy of alopecia areata with diphencyprone. Arch Dermatol 2001;137:1063-8.
Gordon PM, Aldrige RD, McVittie E, Hunter JA. Topical diphencyprone for alopecia areata: evaluation of 48 cases after 30 months’ follow-up. Br J Dermatol 1996;134:869-71.
Cotellessa C, Peris K, Caracciolo E, Mordenti C, Chimenti S. The use of topical diphenylcyclopropenone for the treatment of extensive alopecia areata. J Am Acad Dermatol 2001;44:73-6.
van der Steen PH, van Baar HM, Happle R, Boezeman JB, Perret CM. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol 1991;24:227-30.
van der Steen PH, Boezeman JB, Happle R. Topical immunotherapy for alopecia areata: re-evaluation of 139 cases after an additional follow-up period of 19 months. Dermatology 1992;184:198-201.
Ohlmeier MC, Traupe H, Luger TA, Bohm M. Topical immunotherapy with diphenylcyclopropenone of patients with alopecia areata - a large retrospective study on 142 patients with a self-controlled design. J Eur Acad Dermatol Venereol 2012;26:503-7.
Published
How to Cite
Issue
Section
Categories
License

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














