Histopathological Diagnosis of Alopecia Clinically Relevant to Alopecia Areata
DOI:
https://doi.org/10.33192/smj.v75i2.260753Keywords:
Alopecia areata, scarring alopecia, lichen planopilaris, lupus erythematosus panniculitis, hair disorders, histopathologyAbstract
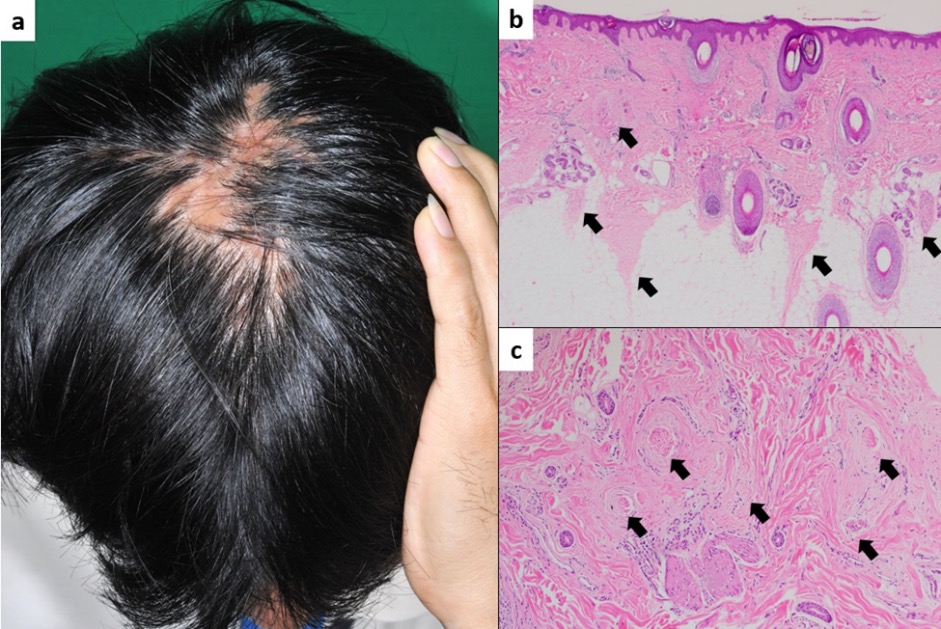
Objective: To study the histopathological diagnosis of alopecia clinically relevant to AA and to compare the histopathology between acute and chronic AA divided by time to onset at three and six months.
Materials & Methods: We conducted a cross-sectional study of 113 patients with typical manifestation of AA. Two scalp biopsies were done horizontally and vertically to confirm diagnosis. Histological findings of AA in the acute group were subsequently compared with the chronic group.
Results: Of the 113 eligible patients, 109 (96.5%) were pathologically diagnosed with AA. Other diagnoses included lichen planopilaris, lupus panniculitis, and unspecified scarring alopecia. The percentage of terminal telogen hairs in the acute group was significantly higher than the chronic group (mean % anagen: % telogen ratio = 21.2%:78.8% vs. 36.0%:64.0%; p = 0.016), while the chronic group had a significantly higher number of follicular streamers (mean ± SD; 2.5 ± 2.2 vs. 3.7 ± 2.6; p = 0.023). The number of vellus hairs significantly increased in the acute group at the six-month onset (p = 0.006). The number of nanogen hairs also increased significantly in the chronic group at both the three- and six-month onset (p = 0.020 and p = 0.007).
Conclusion: Typical manifestations of AA are not always diagnosed as AA. Acute AA has more terminal telogens and vellus hairs, while chronic AA has more follicular streamers and nanogen hairs.
References
Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128(5):702.
Mirzoyev SA, Schrum AG, Davis MDP, Torgerson RR. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134(4):1141-2.
Tan E, Tay YK, Goh CL, Chin Giam Y. The pattern and profile of alopecia areata in Singapore--a study of 219 Asians. Int J Dermatol. 2002;41(11):748-53.
Rajabi F, Drake LA, Senna MM, Rezaei N. Alopecia areata: a review of disease pathogenesis. Br J Dermatol. 2018;179(5):1033-48.
Trueb RM, Dias M. Alopecia Areata: a Comprehensive Review of Pathogenesis and Management. Clin Rev Allergy Immunol. 2018;54(1):68-87.
McElwee K, Freyschmidt-Paul P, Ziegler A, Happle R, Hoffmann R. Genetic susceptibility and severity of alopecia areata in human and animal models. Eur J Dermatol. 2001;11(1):11-6.
Kantor J, Kessler LJ, Brooks DG, Cotsarelis G. Decreased serum ferritin is associated with alopecia in women. J Invest Dermatol. 2003;121(5):985-8.
Seyrafi H, Akhiani M, Abbasi H, Mirpour S, Gholamrezanezhad A. Evaluation of the profile of alopecia areata and the prevalence of thyroid function test abnormalities and serum autoantibodies in Iranian patients. BMC Dermatol. 2005;5:11.
Chu SY, Chen YJ, Tseng WC, Lin MW, Chen TJ, Hwang CY, et al. Comorbidity profiles among patients with alopecia areata: the importance of onset age, a nationwide population-based study. J Am Acad Dermatol. 2011;65(5):949-56.
Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170(6):1299-304.
Lee S, Lee H, Lee CH, Lee WS. Comorbidities in alopecia areata: A systematic review and meta-analysis. J Am Acad Dermatol. 2019;80(2):466-77 e16.
Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, et al. Alopecia areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78(1):1-12.
Messenger AG, McKillop J, Farrant P, McDonagh AJ, Sladden M. British Association of Dermatologists' guidelines for the management of alopecia areata 2012. Br J Dermatol. 2012;166(5):916-26.
Kossard S. Lupus panniculitis clinically simulating alopecia areata. Australas J Dermatol. 2002;43(3):221-3.
Kwong RA, Kossard S. Alopecia areata masquerading as frontal fibrosing alopecia. Australas J Dermatol. 2006;47(1):63-6.
Hoss DM, Grant-Kels JM. Diagnosis: alopecia areata or not? Semin Cutan Med Surg. 1999;18(1):84-90.
Lew BL, Shin MK, Sim WY. Acute diffuse and total alopecia: A new subtype of alopecia areata with a favorable prognosis. J Am Acad Dermatol. 2009;60(1):85-93.
Elston DM. Vertical vs. transverse sections: both are valuable in the evaluation of alopecia. Am J Dermatopathol. 2005;27(4):353-6.
Chen YA, Hsu CK, Lee JY, Yang CC. Linear lupus panniculitis of the scalp presenting as alopecia along Blaschko's lines: a distinct variant of lupus panniculitis in East Asians? J Dermatol. 2012;39(4):385-8.
Chiesa-Fuxench ZC, Kim EJ, Schaffer A, Fett N. Linear lupus panniculitis of the scalp presenting as alopecia along Blaschko's lines: a variant of lupus panniculitis not unique to East Asians. J Dermatol. 2013;40(3):231-2.
Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol. 2012;67(5):1040-8.
Whiting DA. Histopathologic features of alopecia areata: a new look. Arch Dermatol. 2003;139(12):1555-9.
Sperling LC, Lupton GP. Histopathology of non-scarring alopecia. J Cutan Pathol. 1995;22(2):97-114.
Sperling LC, Cowper, S.E., Knopp, E.A. An Atlas of Hair Pathology with Clinical Correlations. second ed: Informa Healthcare; 2012.
Elston DM, McCollough ML, Bergfeld WF, Liranzo MO, Heibel M. Eosinophils in fibrous tracts and near hair bulbs: a helpful diagnostic feature of alopecia areata. J Am Acad Dermatol. 1997;37(1):101-6.
Dy LC, Whiting DA. Histopathology of alopecia areata, acute and chronic: Why is it important to the clinician? Dermatol Ther. 2011;24(3):369-74.
Yoon TY, Lee DY, Kim YJ, Lee JY, Kim MK. Diagnostic usefulness of a peribulbar eosinophilic infiltrate in alopecia areata. JAMA Dermatol. 2014;150(9):952-6.
Published
How to Cite
Issue
Section
Categories
License

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














