Outcomes Comparison of Early versus Late Surfactant Replacement Therapy in Neonates with Respiratory Distress Syndrome
DOI:
https://doi.org/10.33192/smj.v75i5.261175Keywords:
surfactant replacement therapy, respiratory distress syndrome, timing of surfactant, outcomesAbstract
Objective: To compare durations of invasive mechanical ventilator (IMV), other types of ventilator support and neonatal outcomes between neonates who received early versus late surfactant replacement therapy (E-SRT vs. L-SRT).
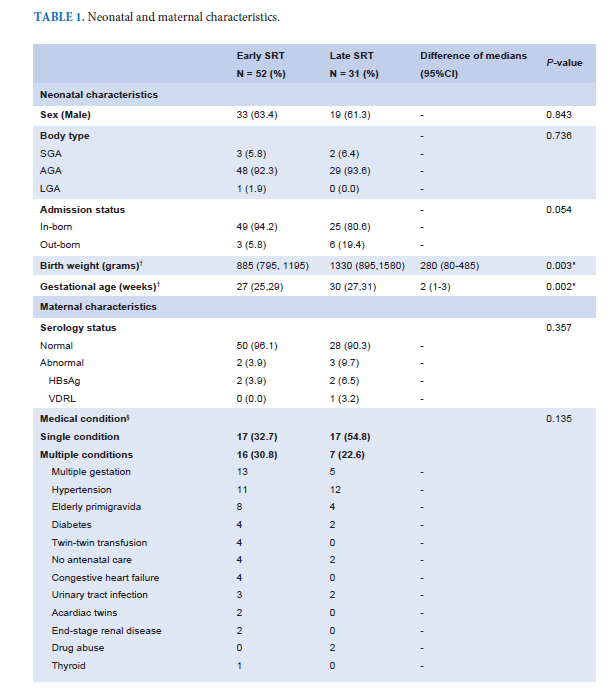
Materials and Methods: This retrospective study included neonates with gestational age (GA) less than 35 weeks or birth weight (BW) less than 2,000 grams, born between January 1, 2017 to December 31, 2021. Neonates who received SRT before 2 hours of life were defined as E-SRT and neonates who received SRT later were defined as L-SRT. Durations of IMV, other types of ventilator support, neonatal outcomes and length of stays were documented.
Results: Eighty-three neonates had received SRT with 52 (62.7%) had E-SRT and 31 (37.3%) had L-SRT. Neonates in E-SRT group had significantly lower GA and BW than neonates in L-SRT group (median GA 27 vs. 30 weeks; p = 0.002 and median BW 885 vs. 1330 grams; p = 0.003) and had longer duration of IMV but not significant (median 19.0 vs. 10.5 days; p = 0.219). There were no significant differences in durations of other types of ventilator support. After adjusted for sex, GA and BW, there were no significant differences in neonatal outcomes between neonates in each group. Ventilator-associated pneumonia (VAP) and septicemia were independent factors associated with prolonged IMV, ventilator supports and length of stays.
Conclusion: Timing of SRT was not associated with duration of IMV. VAP and septicemia were important factors prolonging ventilator durations and length of stays and should be prevented.
Keywords: surfactant replacement therapy, respiratory distress syndrome, timing of surfactant, neonatal outcomes
References
Ainsworth SB. Pathophysiology of Neonatal Respiratory Distress Syndrome. Treat Respir Med. 2005;4(6):423-37.
Jobe AH. 158 - Pathophysiology of Respiratory Distress Syndrome. In: Polin RA, Abman SH, Rowitch DH, Benitz WE, Fox WW, editors. Fetal and Neonatal Physiology (Fifth Edition): Elsevier; 2017.p.1604-19.e2.
Kirsten GF, Kirsten CL, Henning PA, Smith J, Holgate SL, Bekker A, et al. The Outcome of ELBW Infants Treated With NCPAP and InSurE in a Resource-Limited Institution. Pediatrics. 2012;129(4):e952-e9.
Kandraju H, Murki S, Subramanian S, Gaddam P, Deorari A, Kumar P. Early routine versus late selective surfactant in preterm neonates with respiratory distress syndrome on nasal continuous positive airway pressure: a randomized controlled trial. Neonatology. 2013;103(2):148-54.
Kim SM, Park YJ, Chung SH, Choi YS, Kim CH, Bae CW. Early prophylactic versus late selective use of surfactant for respiratory distress syndrome in very preterm infants: a collaborative study of 53 multi-center trials in Korea. J Korean Med Sci. 2014;29(8):1126-31.
Rebello CM, Nacif LF, Deutsch AD, Paes AT. Timing of initial surfactant treatment in very low birth weight newborns. Einstein (Sao Paulo). 2010;8(3):320-4.
Verder H, Robertson B, Greisen G, Ebbesen F, Albertsen P, Lundstrøm K, et al. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish Multicenter Study Group. N Engl J Med. 1994;331(16):1051-5.
Kribs A, Roll C, Göpel W, Wieg C, Groneck P, Laux R, et al. Nonintubated Surfactant Application vs Conventional Therapy in Extremely Preterm Infants: A Randomized Clinical Trial. JAMA Pediatr. 2015;169(8):723-30.
Bancalari E, del Moral T. Bronchopulmonary Dysplasia and Surfactant. Neonatology. 2001;80(Suppl. 1):7-13.
Polin RA, Carlo WA. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014;133(1):156-63.
Ng EH, Shah V. Guidelines for surfactant replacement therapy in neonates. Paediatr Child Health. 2021;26(1):35-49.
Yeh TF, Lin HC, Chang CH, Wu TS, Su BH, Li TC, et al. Early intratracheal instillation of budesonide using surfactant as a vehicle to prevent chronic lung disease in preterm infants: a pilot study. Pediatrics. 2008;121(5):e1310-8.
Yeh TF, Chen CM, Wu SY, Husan Z, Li TC, Hsieh WS, et al. Intratracheal Administration of Budesonide/Surfactant to Prevent Bronchopulmonary Dysplasia. Am J Respir Crit Care Med. 2016;193(1):86-95.
Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J Pediatr. 2018;197:300-8.
Bahadue FL, Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2012;11(11):CD001456.
Cernada M, Brugada M, Golombek S, Vento M. Ventilator-Associated Pneumonia in Neonatal Patients: An Update. Neonatology. 2014;105(2):98-107.
Balany J, Bhandari V. Understanding the Impact of Infection, Inflammation, and Their Persistence in the Pathogenesis of Bronchopulmonary Dysplasia. Front Med (Lausanne). 2015;2:90.
Soll R, Ozek E. Multiple versus single doses of exogenous surfactant for the prevention or treatment of neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2009;(1):CD000141.
Tsakalidis C, Giougki E, Karagianni P, Dokos C, Rallis D, Nikolaidis N. Is there a necessity for multiple doses of surfactant for respiratory distress syndrome of premature infants? Turk J Pediatr. 2012;54(4):368-75.
Willis KA, Weems MF. Hemodynamically significant patent ductus arteriosus and the development of bronchopulmonary dysplasia. Congenit Heart Dis. 2019;14(1):27-32.
Potsiurko S, Dobryanskyy D, Sekretar L. Patent ductus arteriosus, systemic NT-proBNP concentrations and development of bronchopulmonary dysplasia in very preterm infants: retrospective data analysis from a randomized controlled trial. BMC Pediatrics. 2021;21(1):286.
Published
How to Cite
License
Copyright (c) 2023 Siriraj Medical Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














