Adverse Events of Traditional Medicines and Herbal Products in The Thai Health Product Vigilance Center Database and The Ayurved Clinic of Applied Thai Traditional Medicine, Siriraj Hospital
DOI:
https://doi.org/10.33192/smj.v75i5.261512Keywords:
Traditional medicine, Herbal product, Adverse event, Symptom name, Naranjo’s algorithmAbstract
Objective: The aim of this study was to categorize adverse events (AEs) and symptoms related to traditional medicines (TMs) and herbal products (HPs) using the Thai Vigibase program (TVP).
Materials and Methods: TVP collected spontaneous AE reports including causality assessment of medical products in Thailand. For prospective data, Naranjo’s algorithm (NJA) was used to determine the level of causality.
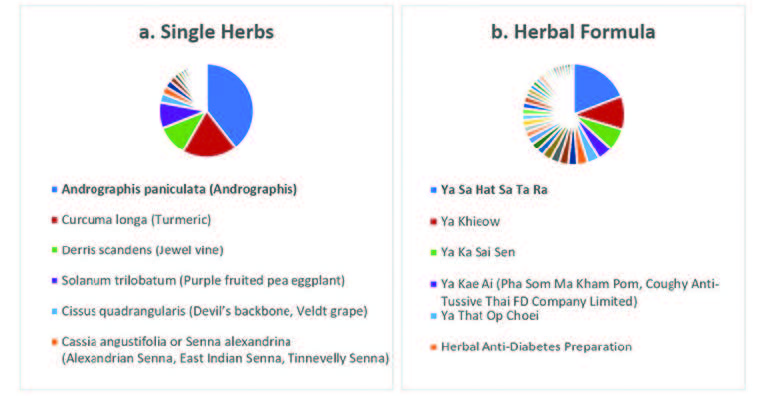
Results: There were a total of 1,133 AE case reports extracted from TVP and featured 1,229 TMs/HPs (310 TMs/HPs names) and 1,592 symptoms (204 symptom names). Andrographis paniculata was the product most frequently linked to AEs, with six cases of confirmed urticaria, 37 probable cases, and 24 possible causalities, 15 patients were given 23 TMs/HPs and this related to 33 AEs. The Ya Hom No.24 Tablets had the most reported AEs at 17.4% with only one causality, which was most probably linked to chest burning pain. There was also one case of herbal decoction relieving menopausal symptoms that was certainly related to chest fullness, feeling hot and cold, suffocation feeling, and sweating increase. Ayurved Siriraj Brand Ya Lom No.65 Pills, also reported one case that was linked to fatigue and drowsiness.
Conclusion: Reports from both data sources found a similar pattern in AE type and TMs/HPs. Naranjo’s algorithm might be one of useful tools to help assess the causality between TMs/HPs and AEs. The results of this study serve as a good reference for causality between TMs/HPs and their AEs for all Thai traditional medicine practitioners.
References
Riewpaiboon A. Increasing herbal product consumption in Thailand. Pharmacoepidemiol Drug Saf. 2006;15(9):683-6.
Thailand Center of Excellence for life Sciences. Three-year strategy plan 2016-2019 แผนยุทธศาสตร์การดำเนินงานในระยะ 3 ปี (2559-2561). 2016 [10 April 2017]; Available from: draft_manuscript.docx 3 ปี (2559-2561).pdf.
World Health Organization (WHO) [Internet]. World alliance for patient safety: WHO draft guidelines for adverse event reporting and learning systems: from information to action. 2020 [cited 2020 Oct 4]. Available from: https://apps.who.int/iris/handle/10665/69797
Australian Government, Department of Health, Therapeutic Goods Administration (TGA) [Internet]. Reporting Adverse Events. 2019 [updated 2019 Oct 30; cited 2020 Oct 4]. Available from: https://www.tga.gov.au/reporting-adverse-events
Administrative Regulation on Reporting and Monitoring of Adverse Drug Reaction. Expedite reporting of adverse events: the agency CFDA (China FDA). Pharma Mirror [Internet]. 2014 [cited 2020 Oct 4]. Available from: https://www.pharmamirror.com/pharmaceutical-articles/expedite-reporting-adverse-events-agency-cfda-china-fda/
World Health Organization (WHO) [Internet]. Pharmacovigilance. 2019 [cited 2020 May 22]. Available from: https://www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmvigi/en/
Australian Government, Department of Health, Therapeutic Goods Administration (TGA) [Internet]. About the TGA. [cited 2020 May 22]. Available from: https://www.tga.gov.au/about-tga
Australian Government, Department of Health, Therapeutic Goods Administration (TGA) [Internet]. Safety information. [cited 2020 May 22]. Available from: https://www.tga.gov.au/safety-information
Zhang L, Wong LY, He Y, Wong IC. Pharmacovigilance in China: current situation, success and challenges. Drug Saf. 2014;37(10):765-70.
Soares Neto JA, Galduroz JC, Marques LC, Kato ET, Macrini T, Rodrigues E. Possible adverse reactions to herbal products: a study with individuals who resort to popular medicine in the city of Diadema, SP, Brazil. Phytother Res. 2014;28(3):405-11.
Susskind M, Thurmann PA, Luke C, Jeschke E, Tabali M, Matthes H, et al. Adverse drug reactions in a complementary medicine hospital: a prospective, intensified surveillance study. Evid Based Complement Alternat Med. 2012;2012:320760.
Patel DN, Low WL, Tan LL, Tan MM, Zhang Q, Low MY, et al. Adverse events associated with the use of complementary medicine and health supplements: an analysis of reports in the Singapore Pharmacovigilance database from 1998 to 2009. Clin Toxicol (Phila). 2012;50(6):481-9.
Health Product Vigilance Center [Internet]. Introduction of Health Product Vigilance Center. 2015 Oct 1 [cited 2020 May 22]. Available from: http://thaihpvc.fda.moph.go.th/thaihvc/Public/Webpage/main.jsf
Sangsuwan C, Udompanthurak S, Vannasaeng S, Thamlikitkul V. Randomized controlled trial of Tinospora crispa for additional therapy in patients with type 2 diabetes mellitus. J Med Assoc Thai 2004;87(5);543-6.
Suwankesawong W, Saokaew S, Permsuwan U, Chaiyakunapruk N. Characterization of hypersensitivity reactions reported among Andrographis paniculata users in Thailand using Health Product Vigilance Center (HPVC) database. BMC Complement Altern Med. 2014;14:515.
Saokaew S, Suwankesawong W, Permsuwan U, Chaiyakunapruk N. Safety of herbal products in Thailand: an analysis of reports in the thai health product vigilance center database from 2000 to 2008. Drug Saf. 2011;34(4):339-50.
Wechwithan S, Suwankesawong W, Sornsrivichai V, McNeil B E, Jiraphongsa C, Chongsuvivatwong V. Signal detection for Thai traditional medicine: examination of national pharmacovigilance data using reporting odds ratio and reported population attributable risk. Regul Toxicol Pharmacol. 2014;70(1):407-12.
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-45.
Zaki SA. Adverse drug reaction and causality assessment scales. Lung India. 2011;28(2):152-3.
IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.
Lindquist M. Documentation grading of ICSRs and improved data quality management in Vigibase [abstract]. Drug Saf 2007;30(10):953.
National Drug Information, Food and Drug Administration of Thailand. National List of Essential Medicines. 2018 Jan 19 [cited 2021 Jan 8]. Available from: http://ndi.fda.moph.go.th/uploads/main_drug_file/20180529192247.pdf
Center of Applied Thai Traditional Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University. Ayurved Siriraj Drug Guideline (Version 1). Bangkok; 2020.p.36.
The Botanical Organization, Ministry of Natural Resource and Environment Thailand. BGO Plant Databases, The Botanical Garden Organization. 2011 [cited 2021 Mar 1]. Available from: http://www.qsbg.org/database/botanic_book full option/search_page.asp
Forest Botany Division, Thailand. Thai Plant Names. 2021 [cited 2021 Mar 1]. Available from: http://www.dnp.go.th/botany/mplant/index.html
New Zealand Medicines and Medical Devices Safety Authority, Ministry of Health, New Zealand Government. Andrographis paniculata – Potential Risk for Allergic Reactions. 2017 Mar 24 [cited 2020 Oct 4]. Available from: https://www.medsafe.govt.nz/safety/EWS/2017/AndrographisPaniculata.asp
Ayurved Thamrong School, Center of Applied Thai Traditional Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University. Drug compounding. Thai Traditional Medicine in the Faculty of Medicine Siriraj Hospital. First edition. Bangkok: Supavanich Press; 2009.p.11-2.
Meyboom RH, Lindquist M, Egberts AC, Edwards IR. Signal selection and follow-up in pharmacovigilance. Drug Saf. 2002;25(6):459-65.
Gryzlak BM, Wallace RB, Zimmerman MB, Nisly NL. National surveillance of herbal dietary supplement exposures: the poison control center experience. Pharmacoepidemiol Drug Saf. 2007;16(9):947-57.
Bunnan K, Chaikomin R, Lohsiriwat S, Chomchai S, Chomchai S, Akarasereenont P. Effect of Ayuraved Siriraj Herbal Recipe “Wattana” on Gastric Emptying Rate. Siriraj Med J. 2020;64(3):89-93. Available from: https://he02.tci-thaijo.org/index.php/sirirajmedj/article/view/244165
Akarasereenont P, Thitilertdecha P, Palo T, Seubnooch P, Wattanarangsan J, Phadungrakwitya R, et al. Chromatographic Fingerprint Development for Quality Assessment of “Ayurved Siriraj Prasachandaeng”, Antipyretic Drug. Siriraj Med J. 2020; 62(1):4-8. Available from: https://he02.tci-thaijo.org/index.php/sirirajmedj/article/view/243638
Seubnooch P, Wattanarangsan J, Thamsermsang O, Booranasubkajorn S, Laohapand T, Akarasereenont P. Chemical Profiling of an Antipyretic Drug, Thai Herbal Harak Formula, by Liquid Chromatography Coupled with Quadrupole Time-of-Flight Mass Spectrometry. Siriraj Med J. 2018;70(2):159-68. Available from: https://he02.tci-thaijo.org/index.php/sirirajmedj/article/view/127514
Wattanarangsan J, Akarasereenont P, Lumlerdkij N, Chotewuttakorn S, Wattanapornchai K, Laohapand T. Ultrasonication Extraction and Solid Phase Extraction Clean-up for Quantification of Aristolochic Acid I in the Thai Herbal Antitussive 3 Formula by High Performance Liquid Chromatography with Photodiode Array Detection. Siriraj Med J. 2018;70(3):227-32. Available from: https://he02.tci-thaijo.org/index.php/sirirajmedj/article/view/129727
Booranasubkajorn S, Kanlaya H, Huabprasert S, Lumlerdkij N, Akarasereenont P, Tripatara P. The Effect of Thai Herbal Ha-Rak Formula (HRF) on LPS-Induced Systemic Inflammation in Wistar Rats. Siriraj Med J. 2017;69(6):356-62. Available from: https://he02.tci-thaijo.org/index.php/sirirajmedj/article/view/106179
Published
How to Cite
Issue
Section
Categories
License
Copyright (c) 2023 Siriraj Medical Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














