Stability of Extemporaneously Prepared Amitriptyline Hydrochloride Topical Preparations for the Treatment of Neuropathic Pain
DOI:
https://doi.org/10.33192/smj.v75i6.261621Keywords:
amitriptyline hydrochloride, neuropathic pain, cold cream, pluronic lecithin organogel, extemporaneous topical preparationsAbstract
Objective: The aim of this study was to investigate the physicochemical and microbiological stability of extemporaneous amitriptyline hydrochloride (AMH) topical preparations for the treatment of neuropathic pain.
Materials and Methods: AMH tablets were triturated to produce fine powders with a mortar and pestle. These powders were levigated and separately incorporated into four compounding bases: hydrophilic petrolatum USP, anionic cream, cold cream USP, and pluronic lecithin organogel (PLO) having the concentration of 2%w/w AMH.
Results: In the in vitro release study, the most significant amount of AMH was released from the PLO, followed by cold cream, anionic cream and hydrophilic petrolatum, respectively; therefore, the compounded AMH in cold cream and AMH in PLO were selected for the evaluation of the in vitro permeation and product stability. The permeation of AMH from PLO across human epidermal membrane was significantly greater than that from the cold cream.
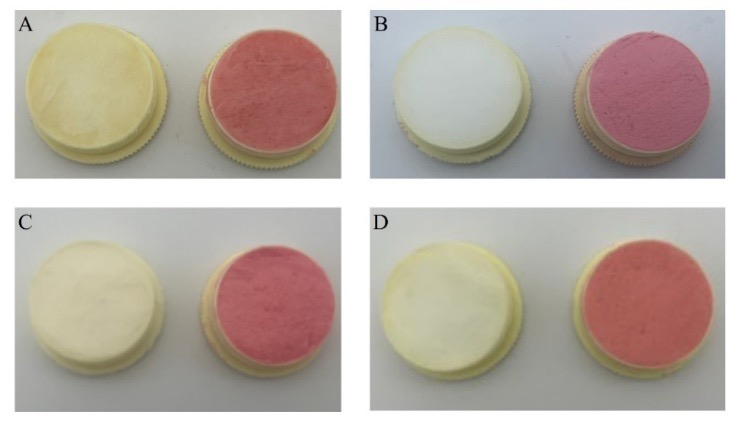
Product stability was characterized as having no remarkable change in color or texture and AMH remaining in the range of 90–110% of the initial concentration quantified by high-performance liquid chromatography. Compounded AMH in cold cream was stable at 2–8 °C and 30 °C for 60 days, and 40 °C for 30 days, whereas compounded AMH in PLO was stable at 30 °C and 40 °C for 14 days. There was no visible microbial growth in any of the samples.
Conclusion: Taken together with the in vitro permeation and product stability studies, the present study suggests that AMH in cold cream could be prepared and used as extemporaneous topical preparations with a beyond-use date of 60 days when kept at 2–8 °C and 30 °C.
References
Loo M. Chronic Pain. In: Loo M, editor. Integrative Medicine for Children. St. Louis, Missouri, USA: Elsevier; 2009. p.238-44.
Jackson TP, Stabile VS, McQueen KAK. The global burden of chronic pain. ASA Monitor. 2014;78(6):24-7.
Gilron C, Watson CP, Cahill CM, Moulin DE. Neuropathic pain: a practical guide for the clinician. Can Med Assoc J. 2006;175(3):265-75.
Park TJ, Shin SY, Suh BC, Suh EK, Lee IS, Kim YS, et al. Differential inhibition of catecholamine secretion by amitriptyline through blockage of nicotinic receptors, sodium channels, and calcium channels in bovine adrenal chromaffin cells. Synapse. 1998;29(3):248-56.
Eisenach JC, Gebhart GF. Intrathecal amitriptyline acts as an N-methyl-D-aspartate receptor antagonist in the presence of inflammatory hyperalgesia in rats. Anesthesiology. 1995;83(5):1046-54.
Traiffort E, Pollard H, Moreau J, Ruat M, Schwartz JC, Martinez-Mir MI, et al. Pharmacological characterization and autoradiographic localization of histamine H2 receptors in human brain identified with [125I]iodoaminopotentidine. J Neurochem. 1992;59(1):290-9.
Brueckle MS, Thomas ET, Seide SE, Pilz M, Gonzalez-Gonzalez AI, Nguyen TS, et al. Adverse drug reactions associated with amitriptyline — protocol for a systematic multiple-indication review and meta-analysis. Syst Rev. 2020;9(1):59-67.
Hansch C, Leo A, Hoekman D. Exploring QSAR: Hydrophobic, electronic, and steric constants In: Hansch C, Leo A, Hoekman D, editors. Exploring QSAR: Hydrophobic, electronic, and steric constants 2. Washington, DC American Chemical Society; 1995.
Liebregts R, Kopsky DJ, Hesselink JMK. Topical amitriptyline in post-traumatic neuropathic pain. J Pain Symptom Manage. 2011;41(4):e6-7.
Kiani J, Nasrollahi SA, Esna-Ashari F, Fallah P, Sajedid F. Amitriptyline 2% cream vs. capsaicin 0.75% cream in the treatment of painful diabetic neuropathy (double blind, randomized clinical trial of efficacy and safety). Iran J Pharm Res. 2015;14(4):1263–8.
Casale R, Symeonidou Z, Bartolo M. Topical treatments for localized neuropathic pain. Curr Pain Headache Rep. 2017;21(3):15-23.
Lynch ME, Clark AJ, Sawynok J, Sullivan MJL. Topical 2% amitriptyline and 1% ketamine in neuropathic pain syndromes: a randomized, double-blind, placebo-controlled trial. Anesthesiology. 2005;103(1):140-6.
Thompson DF, Brooks KG. Systematic review of topical amitriptyline for the treatment of neuropathic pain. J Clin Pharm Ther. 2015;40(5):496-503.
Chantasart D, Chootanasoontorn S, Suksiriworapong J, Li SK. Investigation of pH influence on skin permeation behavior of weak acids using nonsteroidal anti-inflammatory drugs. J Pharm Sci. 2015;104(10):3459-70.
Allen LV. Oinments, creams, and pastes. In: Allen LV, editor. The art, science, and technology of pharmaceutical compounding. 4th ed. Washington, DC, USA: American Pharmacists Association; 2012. p.265-83.
Yang Y, Wang S, Xu H, Sun C, Li X, Zheng JM. Properties of topically applied organogels: rheology and in vitro drug release. Asian J Pharm Sci. 2008;3(4): 175-83.
Mady FM, Essa H, El-Ammawi T, Abdelkader H, Hussein AK. Formulation and clinical evaluation of silymarin pluronic-lecithin organogels for treatment of atopic dermatitis. Drug Des Devel Ther. 2016;10:1101-10.
Bourdon F, Lecoeur M, Leconte L, Ultré V, Kouach M, Odou P, et al. Evaluation of Pentravan®, Pentravan® plus, Phytobase®, Lipovan® and pluronic lecithin organogel for the transdermal administration of antiemetic drugs to treat chemotherapy-induced nausea and vomiting at the hospital. Int J Pharm. 2016;515(1-2):774-87.
Ibrahim MM, Hafez SA, Mahdy MM. Organogels, hydrogels and bigels as transdermal delivery systems for diltiazem hydrochloride. Asian J Pharm Sci. 2013;8:48-57.
International Conference On Harmonisation. Q2(R1) Validation of Analytical Procedures: Text and Methodology. Guidance for Industry. 2005
Chantasart D, Tocanitchart P, Wongrakpanich A, Teeranachaideekul V, Junyaprasert VB. Fabrication and evaluation of Eudragit® polymeric films for transdermal delivery of piroxicam. Pharm Dev Technol. 2018;23(8):771-9.
United States Pharmacopeial Commission. United States Pharmacopeia and National Formulary, USP41-NF36. Rockville, MD: United States Pharmacopeial Convention, Inc. 2018.
Allen LV, Bassani GS, Elder EJ, Parr AF. Strength and stability testing for compounded preparations. Rockville, MD, USA: US Pharmacopeia; 2014. p.1-7.
Sri-in J, Sisan W, Kingkhangphloo P, Jutasompakorn P, Chandranipapongse W, Chatsiricharoenkul S, et al. Stability and sterility of extemporaneously prepared 0.01% atropine ophthalmic solution in artificial tears and balanced salt solution. Siriraj Med J. 2022; 74(2):91-9.
Carafa M, Santucci E, Lucania G. Lidocaine-loaded non-ionic surfactant vesicles: characterization and in vitro permeation studies. Int J Pharm. 2002;231(1):21-32.
Alsaab H, Bonam SP, Bahl D, Chowdhury P, Alexander K, Boddu SH. Organogels in drug delivery: A special emphasis on pluronic lecithin organogels. J Pharm Pharm Sci. 2016;19(2):252-73.
Gupta S. Biocompatible microemulsion systems for drug encapsulation and delivery. Curr Sci. 2011;101(2):174-88.
Frenning G. Theoretical investigation of drug release from planar matrix systems: effects of a finite dissolution rate. J Control Release. 2003;92(3):331-9.
The history of pluronic lecithin organogel: An interview with Marty Jones, BSPharm, FACA, FIACP. Int J Pharm Compound. 2003;7(3):180-6.
Pandey A, Mittal A, Chauhan N, Alam S. Role of surfactants as penetration enhancer in transdermal drug delivery system. J mol Pharm Org Process Res. 2014;2(2):113-22.
Shakshuki A, Yeung P, Agu RU. Compounded topical amitriptyline for neuropathic pain: in vitro release from compounding bases and potential correlation with clinical efficacy. Can J Hosp Pharm. 2020;73(2):133-.40.
Kung CP, Sil BC, Zhang Y, Hadgraft J, Lane ME, Patel B, McCulloch R. Dermal delivery of amitriptyline for topical analgesia. Drug Deliv Transl Res. 2022;12(4):805-15.
Uzaraga I, Gerbis B, Holwerda E, Gillis D, Wai E. Topical amitriptyline, ketamine, and lidocaine in neuropathic pain caused by radiation skin reaction: a pilot study. Support Care Cancer. 2012;20(7):1515-24.
Published
How to Cite
License
Copyright (c) 2023 Siriraj Medical Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














