[RETRACTED ARTICLE] Vaccination Coverage in Patients with Idiopathic Inflammatory Central Nervous System Demyelinating Diseases at Siriraj Hospital, A Single-center Experience
DOI:
https://doi.org/10.33192/smj.v75i8.262732Keywords:
vaccination, idiopathic inflammatory central nervous system demyelinating diseases, multiple sclerosis, neuromyelitis optica spectrum disorder, myelin oligodendrocyte glycoprotein antibody diseaseAbstract
Objective: Individuals with Idiopathic Inflammatory Central Nervous System Demyelinating Diseases (CNS-IIDDs) have an elevated risk for infection. Vaccination is key to reducing infection. This study aimed to determine vaccination coverage, the adverse effects of vaccination, and general vaccination knowledge in the patients.
Methods and Methods: A single-center cross-sectional study in the Multiple Sclerosis Clinic at Siriraj Hospital, Thailand, was performed using the designed questionnaires.
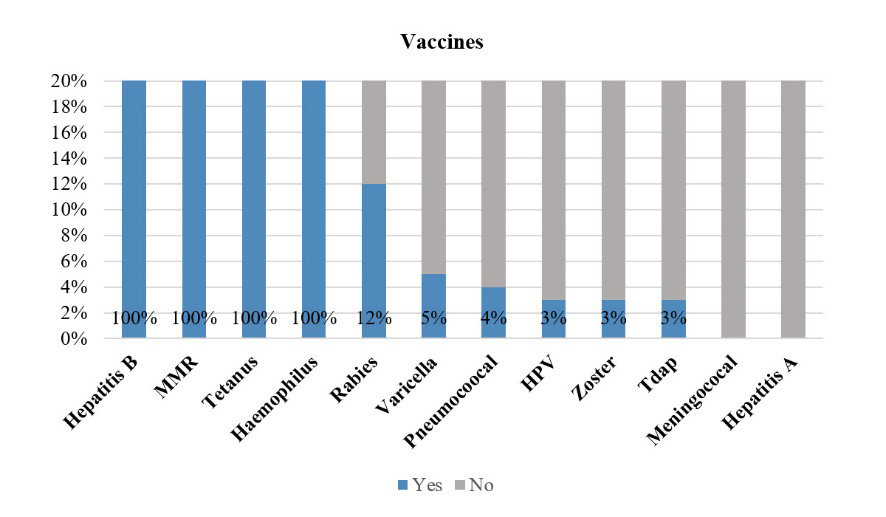
Results: Of 100 participants, 90% were female, with a mean (SD) age of 46.2 (12.9). Overall, all received compulsory vaccine coverage. For optional vaccines, the coverage was lower-than-expected, with rates of 3%, 4%, and 3% for human papilloma virus, pneumococcal, and zoster vaccines, respectively. Only 28% of participants received the 2021/2022 seasonal influenza vaccine. The only factor associated with the uptake of the influenza vaccination was the participants’ health coverage. By asking questions to evaluate general vaccination knowledge, two questions related to vaccination and immuno-suppressive agents received the highest percentage of ‘not sure’ responses.
Conclusion: Vaccination coverage was lower than expected among Thai CNS-IIDDs patients, both for optional and seasonal influenza vaccines. Vaccination in these groups of patients should be encouraged to prevent potential infections.
References
Wijnands JMA, Zhu F, Kingwell E, Fisk JD, Evans C, Marrie RA, et al. Disease-modifying drugs for multiple sclerosis and infection risk: a cohort study. J Neurol Neurosurg Psychiatry. 2018;89(10):1050-6. DOI: https://doi.org/10.1136/jnnp-2017-317493
Swart EC, Mager D, Parekh N, Heyman RA, Henderson R, Munshi K, et al. Infection incidence and management in multiple sclerosis patients after initiating disease-modifying therapy. Mult Scler Relat Disord. 2021;56:103285. DOI: https://doi.org/10.1016/j.msard.2021.103285
Zhao Z, Ma C-L, Gu Z-C, Dong Y, Lv Y, Zhong M-K. Incidence and risk of infection associated with fingolimod in patients with multiple sclerosis: a systematic review and meta-analysis of 8,448 patients from 12 randomized controlled trials. Front Immunol. 2021;12:611711. DOI: https://doi.org/10.3389/fimmu.2021.611711
Montgomery S, Hillert J, Bahmanyar S. Hospital admission due to infections in multiple sclerosis patients. Eur J Neurol. 2013;20(8):1153-60. DOI: https://doi.org/10.1111/ene.12130
Thailand TRCoPo. Recommended adult and elderly immunization schedule 2011 [cited 2022 19 September]. Available from: http://www.rcpt.org/index.php/2012-10-03-16-53-39/category/6-2013-02-02-09-02-52.html?download=56%3A2013-02-02-09-08-53.
Farez MF, Correale J, Armstrong MJ, Rae-Grant A, Gloss D, Donley D, et al. Practice guideline update summary: Vaccine-preventable infections and immunization in multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-94. DOI: https://doi.org/10.1212/WNL.0000000000008157
Marrie RA, Walld R, Bolton JM, Sareen J, Patten SB, Singer A, et al. Uptake of influenza vaccination among persons with inflammatory bowel disease, multiple sclerosis or rheumatoid arthritis: a population-based matched cohort study. CMAJ Open. 2021;9(2):E510-E21. DOI: https://doi.org/10.9778/cmajo.20200105
Marrie RA, Kosowan L, Cutter GR, Fox R, Salter A. Uptake and attitudes about immunizations in people with multiple sclerosis. Neurol Clin Pract. 2021;11(4):327-34. DOI: https://doi.org/10.1212/CPJ.0000000000001099
Sbragia E, Olobardi D, Novi G, Lapucci C, Cellerino M, Boffa G, et al. Vaccinations in patients with multiple sclerosis: a real-world, single-center experience. Hum Vaccin Immunother. 2022;18(6):2099171. DOI: https://doi.org/10.1080/21645515.2022.2099171
Achiron A, Dolev M, Menascu S, Zohar D-N, Dreyer-Alster S, Miron S, et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult Scler. 2021;27(6):864-70. DOI: https://doi.org/10.1177/13524585211003476
Cai H, Zhou R, Jiang F, Zeng Q, Yang H. Vaccination in neuromyelitis optica spectrum disorders: Friend or enemy? Mult Scler Relat Disord. 2022;58:103394. DOI: https://doi.org/10.1016/j.msard.2021.103394
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-73. DOI: https://doi.org/10.1016/S1474-4422(17)30470-2
Wingerchuk D, Banwell B, Bennett J, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-89. DOI: https://doi.org/10.1212/WNL.0000000000001729
López-Chiriboga AS, Majed M, Fryer J, Dubey D, McKeon A, Flanagan EP, et al. Association of MOG-IgG serostatus with relapse after acute disseminated encephalomyelitis and proposed diagnostic criteria for MOG-IgG–associated disorders. JAMA Neurol. 2018;75(11):1355-63. DOI: https://doi.org/10.1001/jamaneurol.2018.1814
Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59(4):499-505. DOI: https://doi.org/10.1212/WNL.59.4.499
Kidd D, Burton B, Plant G, Graham E. Chronic relapsing inflammatory optic neuropathy (CRION). Brain. 2003;126(2):276-84. DOI: https://doi.org/10.1093/brain/awg045
Keja K, Chan C, Hayden G, Henderson RH. Expanded programme on immunization. World Health Stat Q. 1988;41(2):59-63.
Thailand TNIo. Clinical Practice Guidelines: Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorder 2018 [cited 2022 19 September]. Available from: http://neurothai.org/media/news_file/309-9-2561%20Clinical%20practice%20guideline%20MS%20and%20NMOSD.pdf.
Manouchehrinia A, Tanasescu R, Kareem H, Jerca OP, Jabeen F, Shafei R, et al. Prevalence of a history of prior varicella/herpes zoster infection in multiple sclerosis. J Neurovirol. 2017;23(6):839-44. DOI: https://doi.org/10.1007/s13365-017-0569-1
Owusu JT, Prapasiri P, Ditsungnoen D, Leetongin G, Yoocharoen P, Rattanayot J, et al. Seasonal influenza vaccine coverage among high-risk populations in Thailand, 2010–2012. Vaccine. 2015;33(5):742-7. DOI: https://doi.org/10.1016/j.vaccine.2014.10.029
Sirikalyanpaiboon M, Ousirimaneechai K, Phannajit J, Pitisuttithum P, Jantarabenjakul W, Chaiteerakij R, et al. COVID-19 vaccine acceptance, hesitancy, and determinants among physicians in a university-based teaching hospital in Thailand. BMC Infect Dis. 2021;21(1):1174. DOI: https://doi.org/10.1186/s12879-021-06863-5
Downloads
Published
How to Cite
License
Copyright (c) 2023 Siriraj Medical Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














