The Effects of Storage Time at 2–8 Degrees Celsius on the Stability of von Willebrand Factor in Thawed, Platelet-Poor Plasma
DOI:
https://doi.org/10.33192/smj.v75i8.263320Keywords:
Stability, Thawed plasma, VWF:Ag, VWF:CB, VWF:GPIbMAbstract
thawed samples with plasma stored at 2–8 °C for 24–96 hours.
Materials and Methods: Plasma from healthy subjects with normal coagulation times and VWF panels was stored at -20 °C for one week. After thawing (at 0 hours), VWF:antigen (VWF:Ag), VWF:glycoprotein Ib binding assay (VWF:GPIbM), and VWF:collagen binding assay (VWF:CB) were assayed. The remaining plasma was stored at 2–8 °C and assayed at 24, 48, 72, and 96 hours. Differences between levels at baseline and 24, 48, 72, and 96 hours were deemed significant when P was < 0.05.
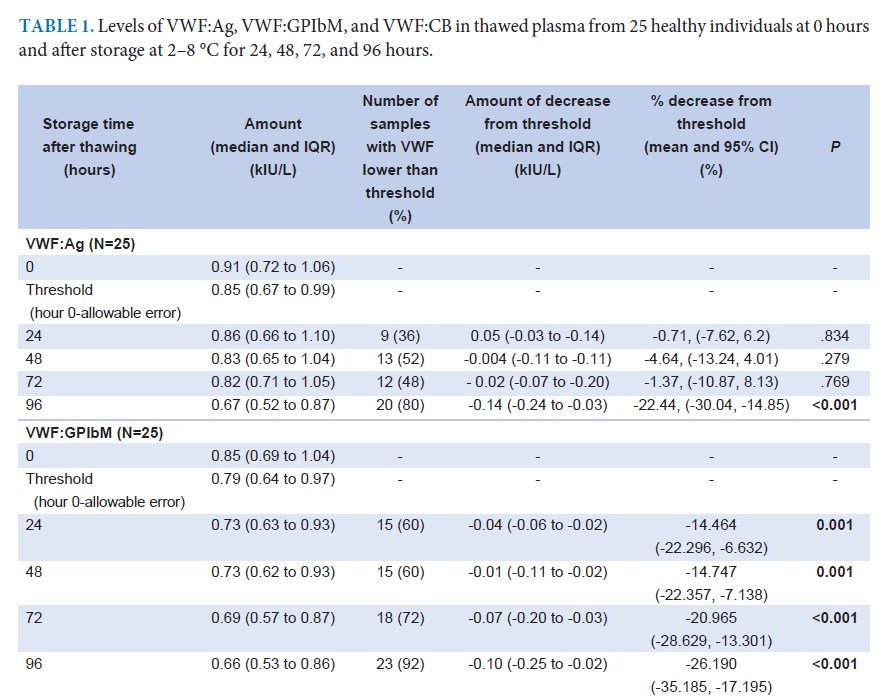
Results: Thirty-five samples were enrolled, with 25 from healthy subjects (VWF:Ag levels > 0.50 kIU/L). Median levels (interquartile range) were as follows: VWF:Ag = 0.91 (0.72–1.06) kIU/L; VWF:GPIbM = 0.85 (0.69–1.04) kIU/L; and VWF:CB = 0.78 (0.62–0.97) kIU/L. VWF:Ag remained stable for 72 hours, while VWF:GPIbM decreased significantly after thawing. VWF:CB declined after 48 hours at 2–8 °C. Similar stability trends were observed in 10 additional samples from VWD patients (VWF:Ag = 0.42 (0.36–0.46) kIU/L).
Conclusion: VWF:Ag and VWF:CB are stable in thawed plasma for 72 hours. VWF:GPIbM is less stable and should not be kept longer than 24 hours. Immediate testing of VWF:GPIbM after thawing is recommended.
References
Ruchutrakool T. von Willebrand Disease in Siriraj Hospital: Where Are We Now? Siriraj Med J. 2010;62(1):42-6.
Corrales-Medina FF, Federici AB, Srivastava A, Dougall A, Millar CM, Roberts JC, et al. A need to increase von Willebrand disease awareness: vwdtest.com - A global initiative to help address this gap. Blood Rev. 2023;58:101018.
Elbatarny M, Mollah S, Grabell J, Bae S, Deforest M, Tuttle A, et al. Normal range of bleeding scores for the ISTH-BAT: adult and pediatric data from the merging project. Haemophilia. 2014;20(6):831-5.
James PD, Connell NT, Ameer B, Di Paola J, Eikenboom J, Giraud N, et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 2021;5(1):280-300.
Kawecki C, Lenting PJ, Denis CV. von Willebrand factor and inflammation. J Thromb Haemost. 2017;15(7):1285-94.
Ward SE, O’Sullivan JM, O’Donnell JS. The relationship between ABO blood group, von Willebrand factor, and primary hemostasis. Blood. 2020;136(25):2864-74.
Magnette A, Chatelain M, Chatelain B, Ten Cate H, Mullier F. Pre-analytical issues in the haemostasis laboratory: guidance for the clinical laboratories. Thromb J. 2016;14:49.
Adcock Funk DM, Lippi G, Favaloro EJ. Quality standards for sample processing, transportation, and storage in hemostasis testing. Semin Thromb Hemost. 2012;38(6):576-85.
Zhao Y, Feng G, Feng L. Effects of pre-analytical storage time, temperature, and freeze-thaw times on coagulation factors activities in citrate-anticoagulated plasma. Ann Transl Med. 2018;6(23):456.
Ingerslev J. A Sensitive ELISA for von Willebrand factor (VWF: Ag). Scand J Clin Lab Invest. 1987;47(2):143-9.
Bodó I, Eikenboom J, Montgomery R, Patzke J, Schneppenheim R, Di Paola J, et al. Platelet-dependent von Willebrand factor activity. Nomenclature and methodology: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(7):1345-50.
Casonato A, Pontara E, Bertomoro A, Sartorello F, Cattini MG, Girolami A. Von Willebrand factor collagen binding activity in the diagnosis of von Willebrand disease: an alternative to ristocetin co-factor activity? Br J Haematol. 2001;112(3):578-83.
Ricós C, Alvarez V, Cava F, García-Lario JV, Hernández A, Jiménez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999;59(7):491-500.
Loeffen R, Kleinegris MC, Loubele ST, Pluijmen PH, Fens D, van Oerle R, et al. Preanalytic variables of thrombin generation: towards a standard procedure and validation of the method. J Thromb Haemost. 2012;10(12):2544-54.
Baker P, Platton S, Gibson C, Gray E, Jennings I, Murphy P, et al. Guidelines on the laboratory aspects of assays used in haemostasis and thrombosis. Br J Haematol. 2020;191(3):347-62.
Suchsland J, Friedrich N, Grotevendt A, Kallner A, Lüdemann J, Nauck M, et al. Optimizing centrifugation of coagulation samples in laboratory automation. Clin Chem Lab Med. 2014;52(8):1187-91.
Zhao Y, Feng G, Zhang J, Gong R, Cai C, Feng L. Effects of preanalytical frozen storage time and temperature on screening coagulation tests and factors VIII and IX activity. Sci Rep. 2017;7(1):12179.
Favaloro EJ, Mehrabani PA. Laboratory assessment of von Willebrand factor: differential influence of prolonged ambient temperature specimen storage on assay results. Haemophilia. 1996;2(4):218-23.
Zürcher M, Sulzer I, Barizzi G, Lämmle B, Alberio L. Stability of coagulation assays performed in plasma from citrated whole blood transported at ambient temperature. Thromb Haemost. 2008;99:416-26.
Gosselin RC, Honeychurch K, Kang HJ, Dwyre DM. Effects of storage and thawing conditions on coagulation testing. Int J Lab Hematol. 2015;37(4):551-9.
Linskens EA, Devreese KMJ. Pre-analytical stability of coagulation parameters in plasma stored at room temperature. Int J Lab Hematol. 2018; 40(3):292-303.
von Heymann C, Keller MK, Spies C, Schuster M, Meinck K, Sander M, et al. Activity of clotting factors in fresh-frozen plasma during storage at 4 degrees C over 6 days. Transfusion. 2009;49(5):913-20.
Buchta C, Felfernig M, Höcker P, Macher M, Körmöczi GF, Quehenberger P, et al. Stability of coagulation factors in thawed, solvent/detergent-treated plasma during storage at 4 degrees C for 6 days. Vox Sang. 2004;87:182-6.
Schoenfeld H, Pruss A, Keller M, Schuster M, Meinck K, Neuner B, et al. Lyophilised plasma: evaluation of clotting factor activity over 6 days after reconstitution for transfusion. J Clin Pathol. 2010;63(8):726-30.
Favaloro EJ, Nair SC, Forsyth CJ. Collection and transport of samples for laboratory testing in von Willebrand’s disease (VWD): time for a reappraisal? Thromb Haemost. 2001;86(6):1589-90.
Favaloro EJ, Soltani S, McDonald J. Potential laboratory misdiagnosis of hemophilia and von Willebrand disorder owing to cold activation of blood samples for testing. Am J Clin Pathol. 2004;122(5):686-92.
Böhm M, Täschner S, Kretzschmar E, Gerlach R, Favaloro EJ, Scharrer I. Cold storage of citrated whole blood induces drastic time-dependent losses in factor VIII and von Willebrand factor: potential for misdiagnosis of haemophilia and von Willebrand disease. Blood Coagul Fibrinolysis. 2006;17(1):39-45.
Published
How to Cite
License
Copyright (c) 2023 Siriraj Medical Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














