Bedaquiline Effect Towards QT Interval in Drug Resistant Tuberculosis (DR-TB): A Systematic Review
DOI:
https://doi.org/10.33192/smj.v75i9.263683Keywords:
Bedaquiline, Drug-Resistant Tuberculosis, QT IntervalAbstract
Objective: Bedaquiline is recommended by World Health Organization (WHO) to treat Drug-Resistant Tuberculosis (DR-TB). Bedaquiline is chosen due to its efficacy and safety in numerous studies. One adverse event that could happen is QT interval prolongation, which increases the risk of Torsade de Pointes (TdP) and leads to death. This study aimed to discuss the knowledge on the effect of bedaquiline on before-after and changes of QT interval.
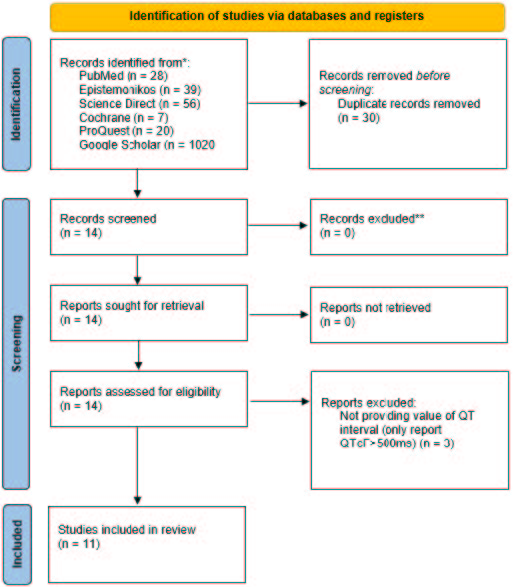
Materials and Methods: This systematic review based on PRISMA guidelines through PubMed, Cochrane, Science Direct, ProQuest, Google Scholar, and Epistemonikos until April 10, 2023. The keywords used was (“Bedaquiline” AND “QT Interval”). We implemented inclusion and exclusion criteria by PICOS framework then assessed the studies by Joanna Briggs Institute (JBI) critical appraisal checklist tools.
Results: From 1.170 articles, eleven articles met the criteria. In total 2449 patients assessed in this study. Most of the studies carried out treatment duration of 6 months. There was a change in the mean QT interval between 11ms to 52.5ms in patients using bedaquiline from the beginning to the end of treatment. The mean QT interval after treatment ranges from 409.7ms – 464.5ms.
Conclusion: The use of bedaquiline requires attention to the ECG before and during therapy. Regular monitoring is necessary to prevent QT prolongation.
References
World Health Organization. WHO consolidated guidelines on tuberculosis Module 4: Treatment Drug-resistant tuberculosis treatment 2022 update. 2022.
Prammananan T. Distribution of Drug-Resistant Genes Among Thai Multidrug-Resistant Mycobacterium Tuberculosis (MDR-TB) Clinical Isolates. Siriraj Med J. 2011;63(3):102-5.
Singh L, Mathibe LJ, Bangalee V. The efficacy of bedaquiline versus kanamycin in multi-drug resistant tuberculosis: A systematic scoping review. Health SA. 2021;26:1708.
Diacon AH, Pym A, Grobusch MP, de los Rios JM, Gotuzzo E, Vasilyeva I, et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med. 2014;371(8):723-32.
Pym AS, Diacon AH, Tang SJ, Conradie F, Danilovits M, Chuchottaworn C, et al. Bedaquiline in the treatment of multidrug- and extensively drug-resistant tuberculosis. Eur Respir J. 2016;47(2):564-74.
Lan Z, Ahmad N, Baghaei P, Barkane L, Benedetti A, Brode SK, et al. Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir Med. 2020;8(4):383-94.
Gaida R, Truter I, Peters CA. Adverse effects of bedaquiline in patients with extensively drug-resistant tuberculosis. S Afr J Infect Dis. 2020;35(1):23.
Hewison C, Khan U, Bastard M, Lachenal N, Coutisson S, Osso E, et al. Safety of Treatment Regimens Containing Bedaquiline and Delamanid in the endTB Cohort. Clin Infect Dis. 2022;75(6):1006-13.
Thomas SHL, Behr ER. Pharmacological treatment of acquired QT prolongation and torsades de pointes. Br J Clin Pharmacol. 2016;81(3):420-7.
Darmayani IGAAPS, Ascobat P, Instiaty I, Sugiri YJR, Sawitri N. Bedaquiline Effect on QT Interval of Drugs-Resistant Tuberculosis Patients: Real World Data. Acta Med Indones. 2022;54(3):389-96.
Khatib R, Sabir FRN, Omari C, Pepper C, Tayebjee MH. Managing drug-induced QT prolongation in clinical practice. Postgrad Med J. 2021;97(1149):452-8.
Primadana V, Yovi I, Estiningsih DS. Bedaquiline Correlation to QT Interval Prolongation in DR-TB Patients. Journal of Respirology. 2022;8(3). Available from: https://doi.org/10.20473/jr.v8-I.3.2022.140-146
Lee HH, Jo KW, Yim JJ, Jeon D, Kang H, Shim TS. Interim treatment outcomes in multidrug-resistant tuberculosis patients treated sequentially with bedaquiline and delamanid. Int J Infect Dis. 2020;98:478-85.
Sutherland HS, Tong AST, Choi PJ, Blaser A, Conole D, Franzblau SG, et al. 3,5-Dialkoxypyridine analogues of bedaquiline are potent antituberculosis agents with minimal inhibition of the hERG channel. Bioorg Med Chem [Internet]. 2019;27(7):1292-307. Available from: https://doi.org/10.1016/j.bmc.2019.02.026
Li J, Yang G, Cai Q, Wang Y, Xu Y, Zhang R, et al. Safety, efficacy, and serum concentration monitoring of bedaquiline in Chinese patients with multidrug-resistant tuberculosis. Int J Infect Dis. 2021;110:179-86.
Dooley KE, Rosenkranz SL, Conradie F, Moran L, Hafner R, von Groote-Bidlingmaier F, et al. QT effects of bedaquiline, delamanid, or both in patients with rifampicin-resistant tuberculosis: a phase 2, open-label, randomised, controlled trial. Lancet Infect Dis. 2021;21(7):975-83.
Katrak S, Lowenthal P, Shen R, True L, Henry L, Barry P. Bedaquiline for multidrug-resistant tuberculosis and QTc prolongation in California. J Clin Tuberc Other Mycobact Dis. 2021;23:100216.
Isralls S, Baisley K, Ngam E, Grant AD, Millard J. QT Interval Prolongation in People Treated With Bedaquiline for Drug-Resistant Tuberculosis Under Programmatic Conditions: A Retrospective Cohort Study. Open Forum Infect Dis. 2021;8(8):ofab413.
Gao JT, Du J, Wu GH, Pei Y, Gao MQ, Martinez L, et al. Bedaquiline-containing regimens in patients with pulmonary multidrug-resistant tuberculosis in China: focus on the safety. Infect Dis Poverty. 2021;10(1):32.
Brust JCM, Gandhi NR, Wasserman S, Maartens G, Omar S V, Ismail NA, et al. Effectiveness and Cardiac Safety of Bedaquiline-Based Therapy for Drug-Resistant Tuberculosis: A Prospective Cohort Study. Clin Infect Dis. 2021;73(11):2083-92.
Ndjeka N, Campbell JR, Meintjes G, Maartens G, Schaaf HS, Hughes J, et al. Treatment outcomes 24 months after initiating short, all-oral bedaquiline-containing or injectable-containing rifampicin-resistant tuberculosis treatment regimens in South Africa: a retrospective cohort study. Lancet Infect Dis. 2022;22(7):1042-51.
Ferlazzo G, Mohr E, Laxmeshwar C, Hewison C, Hughes J, Jonckheere S, et al. Early safety and efficacy of the combination of bedaquiline and delamanid for the treatment of patients with drug-resistant tuberculosis in Armenia, India, and South Africa: a retrospective cohort study. Lancet Infect Dis. 2018;18(5):536-44.
Diacon AH, Pym A, Grobusch MP, de los Rios JM, Gotuzzo E, Vasilyeva I, et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med. 2014;371(8):723-32.
Published
How to Cite
License
Copyright (c) 2023 Siriraj Medical Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














