Incidence of Infection-related Complications and Optimal Saline Irrigation Volume for Preoperative Bowel Preparation to Reduce Postoperative Infections in Hirschsprung’s Disease
DOI:
https://doi.org/10.33192/smj.v75i11.264260Keywords:
Hirschsprung, endorectal pullthrough, complications, irrigation, bowel preparationAbstract
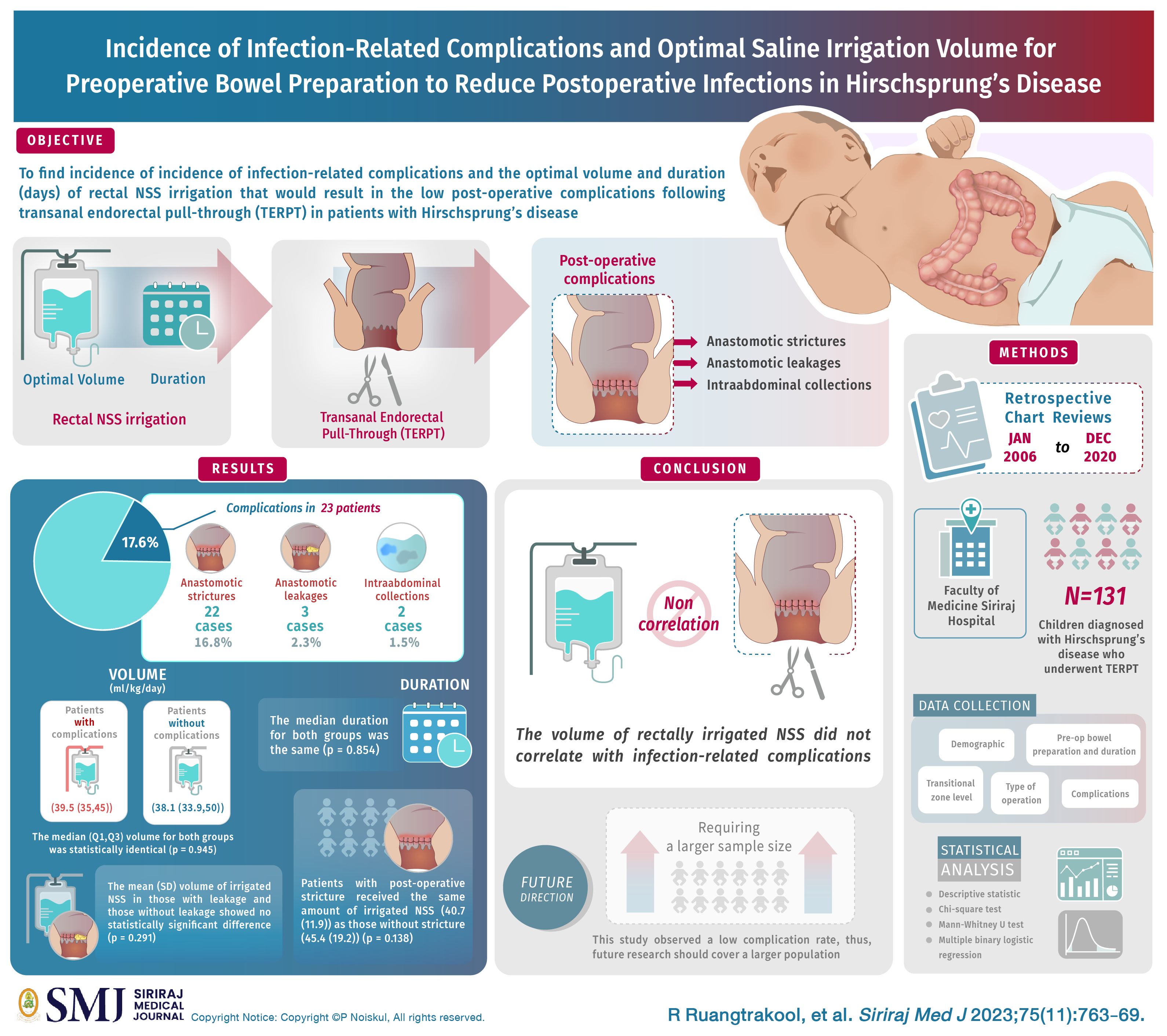
Objective: The purpose of this study was to find incidence of infection-related complications and the optimal volume and duration (days) of rectal NSS irrigation that would result in the low post-operative complications following transanal endorectal pull-through (TERPT) in patients with Hirschprung’s disease.
Materials and Methods: We conducted a retrospective chart reviews of 131 patients diagnosed with Hirschsprung’s disease who underwent TERPT at Siriraj Hospital between January 2006 and December 2020.
Results: Infection-related complications were observed in 23(17.6%) patients, comprising 22(16.8%) cases of anastomotic strictures, 3(2.3%) cases of anastomotic leakages, and 2(1.5%) cases of intraabdominal collections. The median (Q1, Q3) volume of NSS irrigation (ml/kg/day) for those without complications (38.1 (33.9,50)) and those with complications (39.5 (35,45)) was statistically identical (p = 0.945). Similary, the median duration of for both groups was the same (p = 0.854). The mean (SD) volume of irrigated NSS in those with leakage (55.6 (32.7)) and those without leakage (44.3 (17.9)) showed no statistically significant difference (p = 0.291). Patients with post-operative stricture received the same amount of irrigated NSS (40.7 (11.9)) as those without stricture (45.4 (19.2)) (p = 0.138). Similarly, those with hyponatremia received the same amount of irrigated NSS as those without hyponatrema (p = 0.475).
Conclusion: The volume of rectally irrigated NSS did not correlate with infection-related complications such as anastomotic leakage, stricture and intraabdominal collection. However, this study observed a low complication rate, thus, future research should cover a larger population.
References
Spouge D, Baird PA. Hirschsprung disease in a large birth cohort. Teratology. 1985;32:171-7. DOI: https://doi.org/10.1002/tera.1420320204
De la Torre L, Ortega A. Transanal versus open endorectal pull-through for Hirschsprung’s disease. J Pediatr Surg. 2000;35:1630-2. DOI: https://doi.org/10.1053/jpsu.2000.18338
Morowitz MJ, Georgeson KE. Laparoscopic assisted pull-through for Hirschsprung’s disease. In Holcomb GW, Georgeson KE, Rothenberg SS, eds. In: Atlas of Pediatric Laparoscopy and Thoracoscopy, Philadelphia, Elsevier, 2008.p.101-85. DOI: https://doi.org/10.1016/B978-1-4160-3373-8.50023-3
Teitelbaum DH, Cilley RE, Sherman NJ, Bliss D, Uitvlugt ND, Renaud EJ, et al. A decade of experience with the primary pull-through for Hirschsprung disease in the newborn period: A multicenter analysis of outcomes. Ann Surg. 2000;232(3):372-80. DOI: https://doi.org/10.1097/00000658-200009000-00009
The Royal Children's Hospital Melbourne. Bowel washout rectal: clinical guidelines (nursing) [Internet]. Melbourne VIC, Australia [updated 2023 Feb 1; cited 2023 Sep 9]. Available from: https://www.rch.org.au/rchcpg/hospital_clinical_guideline_index/Bowel_washout_rectal/.
UPMC Children’s Hospital of Pittsburgh. Colonic irrigations with metronidazole (Flagyl®) [Internet]. 2023 [cited 2023 Sep 9]. Available from: https://www.chp.edu/our-services/surgery-pediatric/ pediatric-surgery-services-we-offer/colorectal-center-for-children/patient-family-resources/colonic-irrigation#:~:text=Colonic%20Irrigations%20with%20Metronidazole%20(Flagyl%C2%AE).
Hunter A, Mamula P. Bowel preparation for pediatric colonoscopy procedure. J Pediar Gastroenterol. 2010:51(3):254-61. DOI: https://doi.org/10.1097/MPG.0b013e3181eb6a1c
Great Ormond Street Hospital. Bowel washouts using an antegrade colonic enema (ACE) [Internet]. 2019 [cited 2023 Sep 9]. Available from: https://www.gosh.nhs.uk/conditions-and-treatments/procedures-and-treatments/bowel-washouts-using-antegrade-colonic-enema-ace/.
Mungnirandr A. Hirschsprung’s disease: Review article. Siriraj Med J. 2017:69(4):223-7.
Demehri FR, Halaweish IF, Coran AG, Teitelbaum DH. Hirschsprung-associated enterocolitis: pathogenesis, treatment and prevention. Pediatr Surg Int. 2013;29(9):873-81. DOI: https://doi.org/10.1007/s00383-013-3353-1
Parithan P, Chiengkriwate P, Chowchuvech V, Patrapinyokul S, Sangkhathat S. Bowel preparation for pull-through operation in Hirschsprung’s disease. Songkla Med J. 2007;25(5):401-6.
Amsh EA, Lukong CS, Mshelbwata PM, Anumah MA, Gomna A. One-day bowel preparation in children with colostomy using normal saline. Afri J Paed Surg. 2011;8(3):291-3. DOI: https://doi.org/10.4103/0189-6725.91670
Fortuna RS, Weber TR, Tracy Jr. TF, Silen ML, Cradock TV. Critical analysis of the operative treatment of Hirschsprung’s disease. Arch Surg. 1996;131:520-5. DOI: https://doi.org/10.1001/archsurg.1996.01430170066013
Tariq GM, Brereton RJ, Wright VM. Complications of endorectal pull-through for Hirschsprung’s disease. J Pediatr Surg. 1991;26:1202-6. DOI: https://doi.org/10.1016/0022-3468(91)90335-Q
Little DC, Snyder CL. Early and late complications following operative repair of Hirschsprung’s disease. In: Holschneider AM, Puri P, eds. Hirschsprung’s Disease and Allied Disorders, 3rd ed. New York: Springer; 2008.p.375-85. DOI: https://doi.org/10.1007/978-3-540-33935-9_29
Teitelbaum DH, Coran AG. Long-term results and quality of life after treatment of Hirschsprung’s disease and allied disorders. In: Holschneider AM, Puri P, eds. Hirschsprung’s Disease and Allied Disorders, 3rd ed. New York: Springer; 2008.p.389-97.
Engum SA, Grosfeld JL. Long-term results of treatment of Hirschsprung’s disease. Semin Pediatr Surg. 2004;13:273-85. DOI: https://doi.org/10.1053/j.sempedsurg.2004.10.015
Ruangtrakool R, Krajangjit P. Early surgical complications following transanal endorectal pull-through for Hirschsprung’s Disease. Siriraj Med J. 2023;75(6):445-53. DOI: https://doi.org/10.33192/smj.v75i6.261710
Published
How to Cite
License
Copyright (c) 2023 Siriraj Medical Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














