Efficacy of Atropine Eye Drops for Suppressing Myopia Progression in Thai Children
DOI:
https://doi.org/10.33192/smj.v75i11.264383Keywords:
Myopia, Atropine Eye DropsAbstract
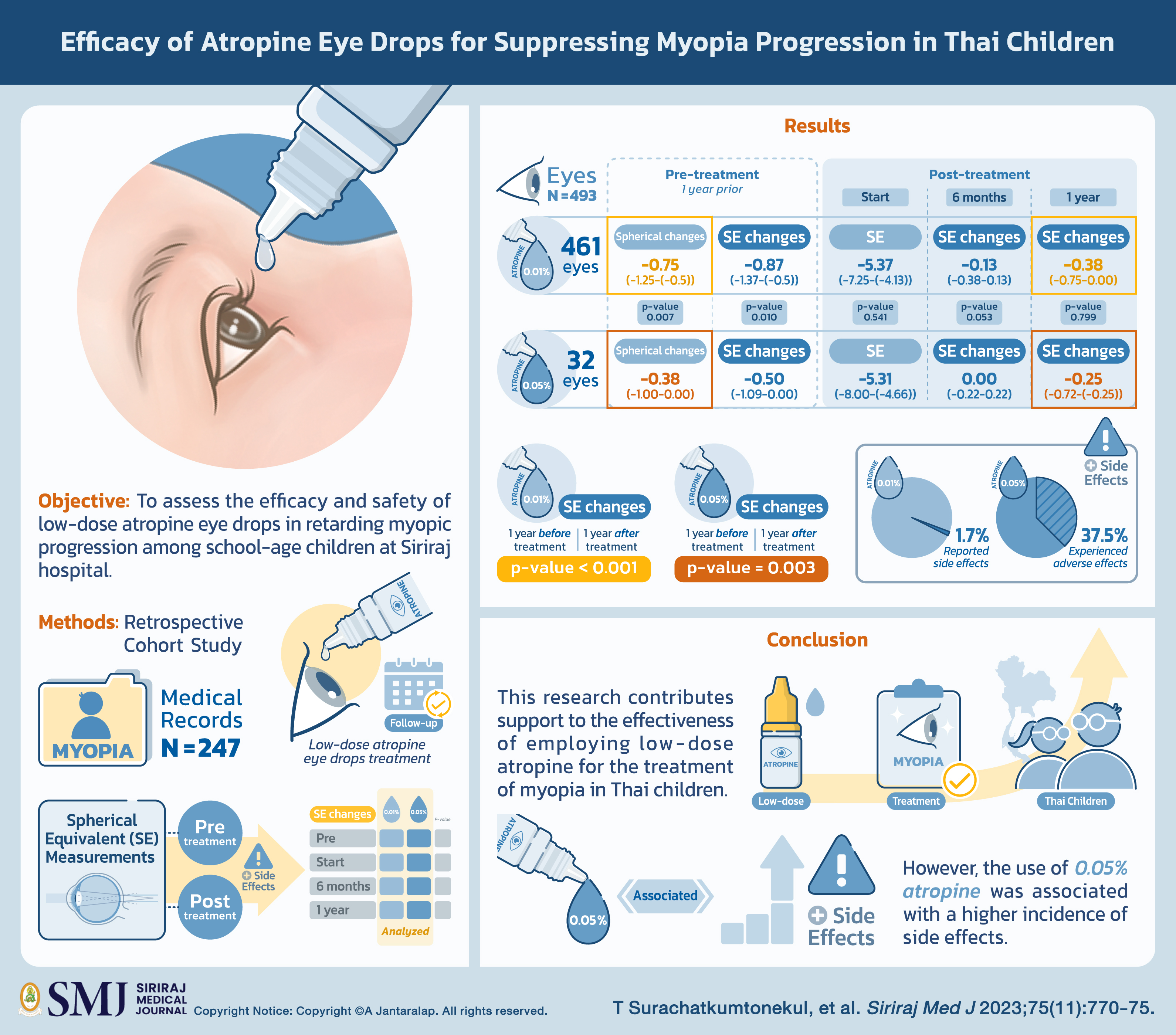
Objective: This retrospective cohort study aimed to assess the efficacy and safety of low-dose atropine eye drops in retarding myopic progression among school-age children at Siriraj hospital.
Materials and Methods: The medical records of 248 myopia-diagnosed patients were reviewed. All patients were received low-dose atropine eye drops and had at least one follow-up visit within 1 year after the treatment initiation. Spherical equivalent (SE) measurements were collected at pre- and post-treatment visits, as well as any reported side effects. Comparing the SE changes observed between the pre- and post-treatment periods, as well as between the two different concentrations of atropine was analyzed.
Results: A total of 495 eyes were analyzed, with 461 eyes receiving 0.01% atropine eye drops and 32 eyes being administered 0.05%. The demographic data between two groups showed no significant difference. The comparison of SE change one year prior to and one year after treatment in the 0.01% and 0.05% group yielded a p-value of less than 0.001 and 0.003, respectively, (SE change are -0.38 (-0.75-0.00) and -0.25 (-0.72-(-0.25)) in the 0.01% and 0.05% group, respectively). However, the between-group comparison of SE change at 6 months and 1 year showed no significant difference. Regarding side effects, one-third of the eyes in the 0.05% group (37.5%) experienced adverse effects while only eight eyes (1.7%) in the 0.01% group reported side effects.
Conclusion: This research contributes support to the effectiveness of employing low-dose atropine for the treatment of myopia in Thai children. Nonetheless, it is worth noting that the use of 0.05% atropine was associated with a higher incidence of side effects.
References
Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-42. DOI: https://doi.org/10.1016/j.ophtha.2016.01.006
Matsumura S, Ching-Yu C, Saw S-M. Global Epidemiology of Myopia. In: Ang M, Wong TY, editors. Updates on Myopia: A Clinical Perspective. Singapore: Springer Singapore; 2020. p. 27-51. DOI: https://doi.org/10.1007/978-981-13-8491-2_2
Dolgin E. The myopia boom. Nature. 2015;519(7543):276-8. DOI: https://doi.org/10.1038/519276a
Zhang X, Cheung SSL, Chan HN, Zhang Y, Wang YM, Yip BH, et al. Myopia incidence and lifestyle changes among school children during the COVID-19 pandemic: a population-based prospective study. Br J Ophthalmol. 2022;106(12):1772-8. DOI: https://doi.org/10.1136/bjophthalmol-2021-319307
Xu L, Ma Y, Yuan J, Zhang Y, Wang H, Zhang G, et al. COVID-19 Quarantine Reveals That Behavioral Changes Have an Effect on Myopia Progression. Ophthalmology. 2021;128(11):1652-4. DOI: https://doi.org/10.1016/j.ophtha.2021.04.001
Pan CW, Dirani M, Cheng CY, Wong TY, Saw SM. The age-specific prevalence of myopia in Asia: a meta-analysis. Optom Vis Sci. 2015;92(3):258-66. DOI: https://doi.org/10.1097/OPX.0000000000000516
Theophanous C, Modjtahedi BS, Batech M, Marlin DS, Luong TQ, Fong DS. Myopia prevalence and risk factors in children. Clin Ophthalmol. 2018;12:1581-7. DOI: https://doi.org/10.2147/OPTH.S164641
Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25(5):381-91. DOI: https://doi.org/10.1111/j.1475-1313.2005.00298.x
Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106(10):2010-5. DOI: https://doi.org/10.1016/S0161-6420(99)90416-5
Iwase A, Araie M, Tomidokoro A, Yamamoto T, Shimizu H, Kitazawa Y. Prevalence and causes of low vision and blindness in a Japanese adult population: the Tajimi Study. Ophthalmology. 2006;113(8):1354-62. DOI: https://doi.org/10.1016/j.ophtha.2006.04.022
Huang J, Wen D, Wang Q, McAlinden C, Flitcroft I, Chen H, et al. Efficacy Comparison of 16 Interventions for Myopia Control in Children: A Network Meta-analysis. Ophthalmology. 2016;123(4):697-708. DOI: https://doi.org/10.1016/j.ophtha.2015.11.010
Tran HDM, Tran YH, Tran TD, Jong M, Coroneo M, Sankaridurg P. A Review of Myopia Control with Atropine. J Ocul Pharmacol Ther. 2018;34(5):374-379. DOI: https://doi.org/10.1089/jop.2017.0144
Zhang Z, Zhou Y, Xie Z, Chen T, Gu Y, Lu S, Wu Z. The effect of topical atropine on the choroidal thickness of healthy children. Sci Rep. 2016;6:34936. DOI: https://doi.org/10.1038/srep34936
Barathi VA, Beuerman RW. Molecular mechanisms of muscarinic receptors in mouse scleral fibroblasts: Prior to and after induction of experimental myopia with atropine treatment. Mol Vis. 2011;17:680-92.
Barathi VA, Weon SR, Beuerman RW. Expression of muscarinic receptors in human and mouse sclera and their role in the regulation of scleral fibroblasts proliferation. Mol Vis. 2009;15:1277-93.
Gallego P, Martinez-Garcia C, Perez-Merino P, Ibares-Frías L, Mayo-Iscar A, Merayo-Lloves J. Scleral changes induced by atropine in chicks as an experimental model of myopia. Ophthalmic Physiol Opt. 2012;32:478–84 DOI: https://doi.org/10.1111/j.1475-1313.2012.00940.x
Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285-91. DOI: https://doi.org/10.1016/j.ophtha.2006.05.062
Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012;119(2):347-54. DOI: https://doi.org/10.1016/j.ophtha.2011.07.031
Clark TY, Clark RA. Atropine 0.01% Eyedrops Significantly Reduce the Progression of Childhood Myopia. J Ocul Pharmacol Ther. 2015;31(9):541-5. DOI: https://doi.org/10.1089/jop.2015.0043
Hieda O, Hiraoka T, Fujikado T, Ishiko S, Hasebe S, Torii H, et al. Efficacy and safety of 0.01% atropine for prevention of childhood myopia in a 2-year randomized placebo-controlled study. Jpn J Ophthalmol. 2021;65(3):315-25. DOI: https://doi.org/10.1007/s10384-021-00822-y
Yam JC, Jiang Y, Tang SM, Law AKP, Chan JJ, Wong E, et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmology. 2019;126(1):113-24. DOI: https://doi.org/10.1016/j.ophtha.2018.05.029
Yam JC, Li FF, Zhang X, Tang SM, Yip BHK, Kam KW, Ko ST, Young AL, Tham CC, Chen LJ, Pang CP. Two-Year Clinical Trial of the Low-Concentration Atropine for Myopia Progression (LAMP) Study: Phase 2 Report. Ophthalmology. 2020;127(7):910-9. DOI: https://doi.org/10.1016/j.ophtha.2019.12.011
Gong Q, Janowski M, Luo M, Wei H, Chen B, Yang G, et al. Efficacy and Adverse Effects of Atropine in Childhood Myopia: A Meta-analysis. JAMA Ophthalmol. 2017;135(6):624-30. DOI: https://doi.org/10.1001/jamaophthalmol.2017.1091
Bedrossian RH. Treatment of progressive myopia with atropine. Presented at: The XX International Congress of Ophthalmology; 14–19 August 1966; Munich, Germany.
Gimbel HV. The control of myopia with atropine. Can J Ophthalmol. 1973;8(4):527-32.
Yen MY, Liu JH, Kao SC, Shiao CH. Comparison of the effect of atropine and cyclopentolate on myopia. Ann Ophthalmol. 1989;21(5):180-2, 187.
Fan DS, Lam DS, Chan CK, Fan AH, Cheung EY, Rao SK. Topical atropine in retarding myopic progression and axial length growth in children with moderate to severe myopia: a pilot study. Jpn J Ophthalmol. 2007;51(1):27-33. DOI: https://doi.org/10.1007/s10384-006-0380-7
Sri-in J, Sisan W, Kingkhangphloo P, Jutasompakorn P, Chandranipapongse W, Chatsiricharoenkul S, et al. Stability and Sterility of Extemporaneously Prepared 0.01% Atropine Ophthalmic Solution in Artificial Tears and Balanced Salt Solution. Siriraj Med J [Internet]. 2022 Feb. 1 [cited 2023 Sep. 10];74(2):91-9. DOI: https://doi.org/10.33192/Smj.2022.12
Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116(3):572-9. DOI: https://doi.org/10.1016/j.ophtha.2008.10.020
Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol. 2014;157(2):451-7.e1. DOI: https://doi.org/10.1016/j.ajo.2013.09.020
Chia A, Lu QS, Tan D. Five-Year Clinical Trial on Atropine for the Treatment of Myopia 2: Myopia Control with Atropine 0.01% Eyedrops. Ophthalmology. 2016;123(2):391-9. DOI: https://doi.org/10.1016/j.ophtha.2015.07.004
Pineles SL, Kraker RT, VanderVeen DK, Hutchinson AK, Galvin JA, Wilson LB, et al. Atropine for the Prevention of Myopia Progression in Children: A Report by the American Academy of Ophthalmology. Ophthalmology. 2017;124(12):1857-66. DOI: https://doi.org/10.1016/j.ophtha.2017.05.032
Kaiti R, Shyangbo R, Sharma IP. Role of Atropine in the control of Myopia Progression- A Review. Beyoglu Eye J. 2022;7(3):157-66. DOI: https://doi.org/10.14744/bej.2022.07742
Published
How to Cite
License
Copyright (c) 2023 Siriraj Medical Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














