Clinical Efficacy of Andrographis paniculata Extracted Scrub Compared With 4% Chlorhexidine Scrub in Burn Wounds: A Prospective Randomized Controlled Trial
DOI:
https://doi.org/10.33192/smj.v75i11.264451Keywords:
Andrographis paniculata, wound cleansing, second degree burnAbstract
Objective: The primary objective of this study is to compare the healing rate between AP soap and 4% Chlorhexidine solution in superficial second-degree burn wounds. The secondary objectives include the analgesic effect and moisturization of these two products.
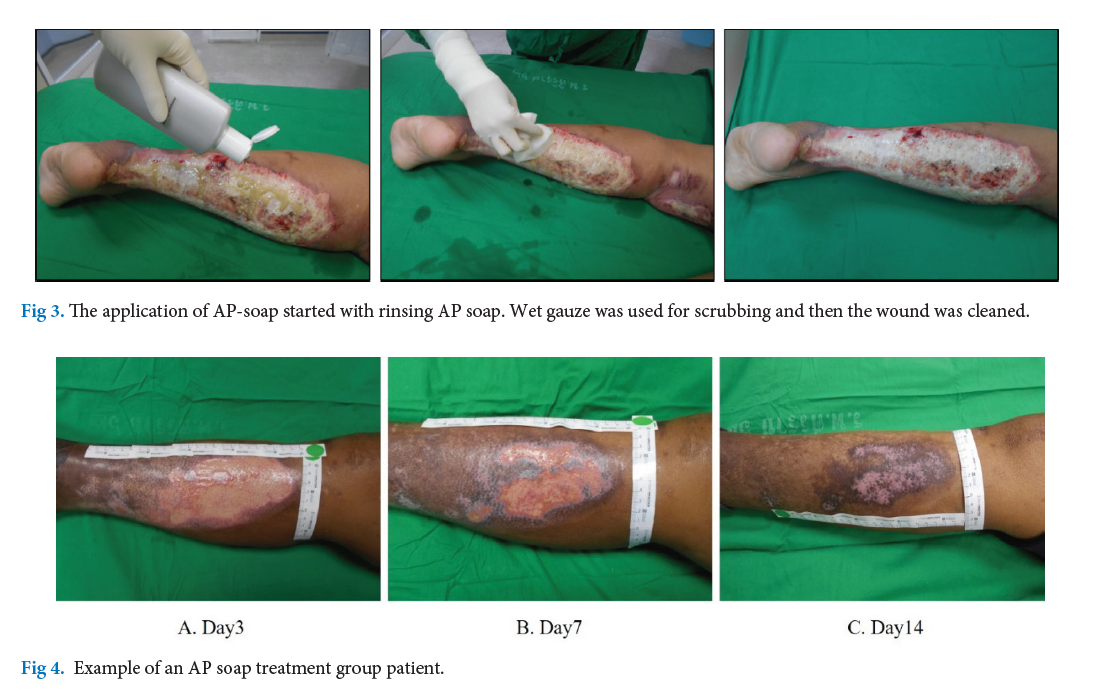
Materials and Methods: Data was collected between 2019 and 2021. Patients aged 18 years and above, with superficial second-degree burns including at least 20% of TBSA, and admitted to the Burn Unit within 24 hours of injury were included. They were randomly assigned to two groups: Andrographis paniculata with Perilla oil liquid soap group (AP group) and 4% Chlorhexidine group (control group). The measurements included percentage of epithelialization, pain score during wound cleansing, itching score after wound cleansing, and dry skin specified symptoms. All patients received standard care for burn wound treatment.
Results: A total enrollment was 23 patients in this study (12 in the AP group and 11 in the control group). The median age was 38.5 years. There were no statistically significant differences in age, %TBSA, and initial wound size between both groups (p > 0.05). Although the healing time was similar in both groups, (18.5 vs. 20.1, p=0.347), the AP group had a significantly lower pain score than the control group (4.7 vs. 5.4, p=0.020). Moreover, the AP group demonstrated significant improvements in itching score and SRRC score at 14 days compared to the control group (5.1 vs. 6.0, p 0.039 and 1.08 vs. 1.55, p 0.020, respectively). There were no adverse effects during this study.
Conclusion: Patients treated with Andrographis paniculata with Perilla oil liquid soap experienced less pain and better moisturization compared to those treated with the standard 4% chlorhexidine solution, while achieving a comparable healing rate. A future large-scale prospective trial is recommended.
References
Matthews JB, Mandell SP, Gibran NS. Burns. In: Brunicardi FC, editor. Schwartz’s Principle of Surgery. 1. 11 ed. United States: McGraw-Hill Educaion; 2019. p. 251-69.
Courtney M. Townsend KLM. Sabiston Textbook of Surgery. 20 ed. Philadelphia: Elsevier Inc; 2017. p. 505-31.
Hayek S, El Khatib A, Atiyeh B. Burn wound cleansing - a myth or a scientific practice. Ann Burns Fire Disasters. 2010;23(1):19-24.
Guidelines IBP, Atiyeh B, Barret JP, Greenfield E, Dahai PH, Duteille PF, et al. Best Practice Guidelines : Effective skin and wound management of non- complex burns. Wounds International. 2014.
Chuntrasakul C, Muangman P, Benjathanung R, Suvanchote S, Boonpamee S, Jantarapakdee S, et al. Clinical Experience of ActicoatTM Treatment in Extensive Burn Wounds. Siriraj Med J. 2007;59(2):47-51.
Falanga V. Wound bed preparation: science applied to practice. In: Calne S, editor. European Wound Management Association position document: Wound bed preparation in practice. London: MEP Ltd; 2004.
D'Avignon LC, Saffle JR, Chung KK, Cancio LC. Prevention and management of infections associated with burns in the combat casualty. J Trauma. 2008;64(3 Suppl):S277-86. DOI: https://doi.org/10.1097/TA.0b013e318163c3e4
DeSanti L. Pathophysiology and Current Management of Burn Injury. 2005;18(6):323-32. DOI: https://doi.org/10.1097/00129334-200507000-00013
Hess CT. Checklist for factors affecting wound healing. Adv Skin Wound Care. 2011;24(4):192. DOI: https://doi.org/10.1097/01.ASW.0000396300.04173.ec
Liu JX, Werner J, Kirsch T, Zuckerman JD, Virk MS. Cytotoxicity evaluation of chlorhexidine gluconate on human fibroblasts, myoblasts, and osteoblasts. J Bone Jt Infect. 2018;3(4):165-72. DOI: https://doi.org/10.7150/jbji.26355
2002 WHOG. WHO monographs on selected medicinal plants. World Health Organization. 2002;2:12-24.
Trivedi NP, Rawal UM, Patel BP. Hepatoprotective effect of andrographolide against hexachlorocyclohexane-induced oxidative injury. Integr Cancer Ther. 2007;6(3):271-80. DOI: https://doi.org/10.1177/1534735407305985
Chang H-MAB, Paul Pui-Hay, A Yao, Sih-Cheng, A Wang, Lai-Ling, A Yeung, Shem Chang-Shing. Pharmacology and Applications of Chinese Materia Medica. World Scientific, Singapore, 1986.
Farnsworth NR, Krause EC, Bolton JL, Pauli GF, van Breemen RB, Graham JG. The University of Illinois at Chicago/National Institutes of Health Center for Botanical Dietary Supplements Research for Women's Health: from plant to clinical use. Am J Clin Nutr. 2008;87(2):504S-8S. DOI: https://doi.org/10.1093/ajcn/87.2.504S
Koteswara Rao Y, Vimalamma G, Rao CV, Tzeng YM. Flavonoids and andrographolides from Andrographis paniculata. Phytochemistry. 2004;65(16):2317-21. DOI: https://doi.org/10.1016/j.phytochem.2004.05.008
Zou W, Xiao Z, Wen X, Luo J, Chen S, Cheng Z, et al. The anti-inflammatory effect of Andrographis paniculata (Burm. f.) Nees on pelvic inflammatory disease in rats through down-regulation of the NF-kappaB pathway. BMC Complement Altern Med. 2016;16(1):483. DOI: https://doi.org/10.1186/s12906-016-1466-5
Jarukamjorn K, Nemoto N. Pharmacological Aspects of Andrographis paniculata on Health and Its Major Diterpenoid Constituent Andrographolide. Journal of Health Science. 2008;54:370-81. DOI: https://doi.org/10.1248/jhs.54.370
Hidalgo MA, Romero A, Figueroa J, Cortes P, Concha, II, Hancke JL, et al. Andrographolide interferes with binding of nuclear factor-kappaB to DNA in HL-60-derived neutrophilic cells. Br J Pharmacol. 2005;144(5):680-6. DOI: https://doi.org/10.1038/sj.bjp.0706105
Jayakumar T, Hsieh CY, Lee JJ, Sheu JR. Experimental and Clinical Pharmacology of Andrographis paniculata and Its Major Bioactive Phytoconstituent Andrographolide. Evid Based Complement Alternat Med. 2013;2013:846740. DOI: https://doi.org/10.1155/2013/846740
Singha PK, Roy S, Dey S. Antimicrobial activity of Andrographis paniculata. Fitoterapia. 2003;74(7):692-4. DOI: https://doi.org/10.1016/S0367-326X(03)00159-X
Tsuchiya H, Sato M, Miyazaki T, Fujiwara S, Tanigaki S, Ohyama M, et al. Comparative study on the antibacterial activity of phytochemical flavanones againt methicillin-resistant Staphylococcus aureus. J Ethnopharmacol. 1996;50(1):27-34. DOI: https://doi.org/10.1016/0378-8741(96)85514-0
Asif M. Health effects of omega-3,6,9 fatty acids: Perilla frutescens is a good example of plant oils. Orient Pharm Exp Med. 2011;11(1):51-9. DOI: https://doi.org/10.1007/s13596-011-0002-x
Tianwattanatada S, Namviriyachote N, Chinaroonchai K, Owattanapanich N, Nair HKR, Muangman P. Clinical Efficacy Test of Polyester Dressing Containing Herbal Extracts and Silver Sulfadiazine Cream Compared with Silver Sulfadiazine Cream in Healing Burn Wounds: A Prospective Randomized Controlled Trial. Siriraj Med J. 2021;73(11):752-7. DOI: https://doi.org/10.33192/Smj.2021.97
Abdel-Sayed P, Tornay D, Hirt-Burri N, de Buys Roessingh A, Raffoul W, Applegate LA. Implications of chlorhexidine use in burn units for wound healing. Burns. 2020;46(5):1150-6. DOI: https://doi.org/10.1016/j.burns.2019.12.008
Al-Bayaty FH, Abdulla MA, Hassan MIA, Ali HM. Effect of Andrographis paniculata leaf extract on wound healing in rats. Nat Prod Res. 2012;26(5):423-9. DOI: https://doi.org/10.1080/14786419.2010.496114
Norman AT, Judkins KC. Pain in the patient with burns. Continuing Education in Anaesthesia Critical Care & Pain. 2004;4(2):57-61. DOI: https://doi.org/10.1093/bjaceaccp/mkh016
Reich A, Heisig M, Phan NQ, Taneda K, Takamori K, Takeuchi S, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol. 2012;92(5):497-501. DOI: https://doi.org/10.2340/00015555-1265
Serup J. EEMCO guidance for the assessment of dry skin (xerosis) and ichthyosis: clinical scoring systems. Skin Res Technol. 1995;1(3):109-14. DOI: https://doi.org/10.1111/j.1600-0846.1995.tb00029.x
Al-Kotb H, Abdel-Aziz H. Effect of Standardized Skin Care Guidelines on Skin Dryness among Elderly People at Ismailia City. Journal of Nursing and Health Science. 2017;6:12-8.
Published
How to Cite
License
Copyright (c) 2023 Siriraj Medical Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














