Assessing Low-Concentration Atropine in Myopia Progression: A Systematic Review
DOI:
https://doi.org/10.33192/smj.v75i12.265388Keywords:
Low-Concentration Atropine Eye Drop, Myopia, ChildrenAbstract
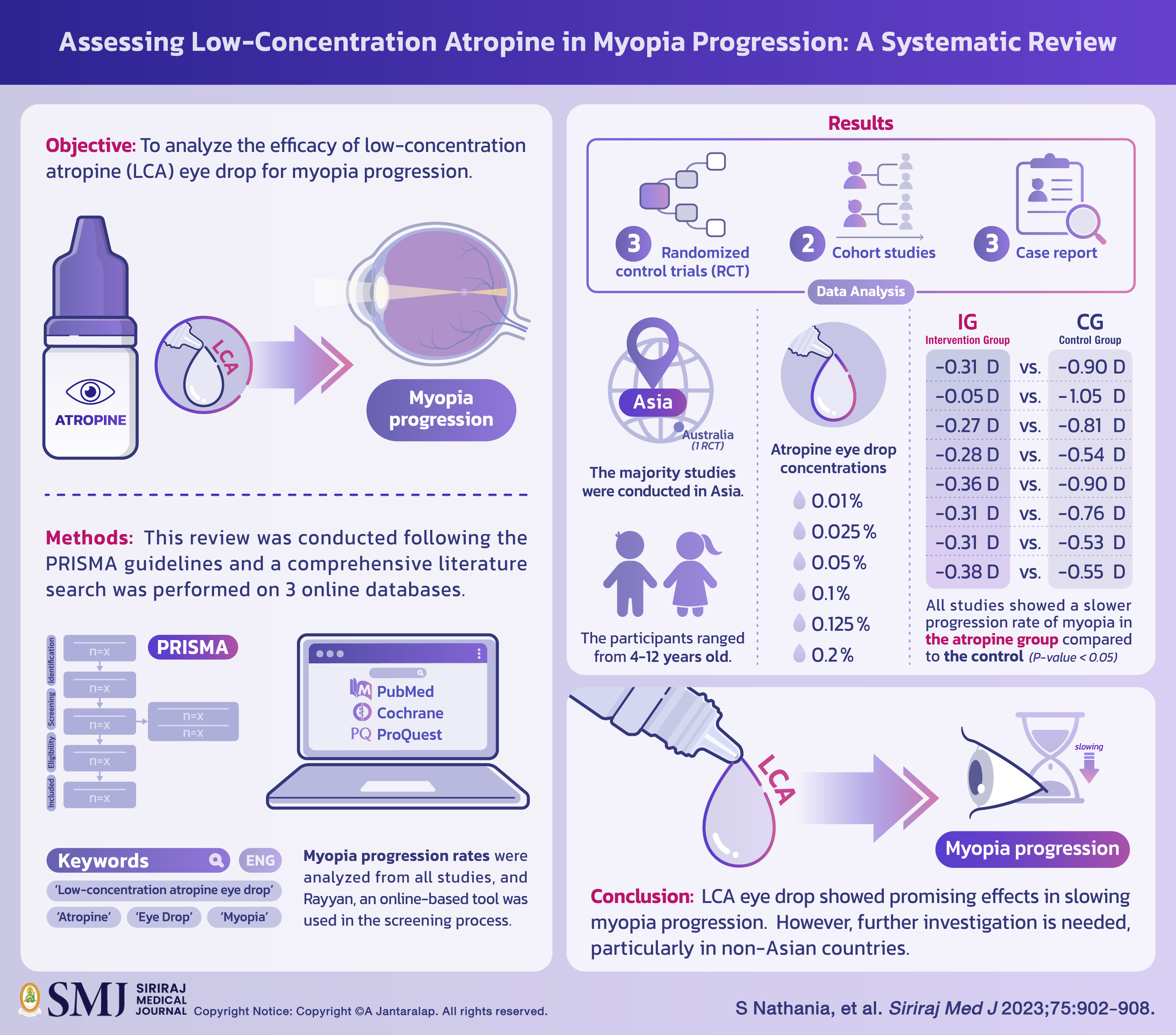
Objective: Low-concentration atropine (LCA) eye drop is used as a promising treatment for the management of myopia but its effectiveness has not been widely evaluated. Therefore, this study aimed to analyze the efficacy of LCA eye drop for myopia progression.
Materials and Methods: This review was conducted following the PRISMA guidelines and a comprehensive literature search was performed on 3 online databases including PubMed, Cochrane, and ProQuest. The keywords used included ‘Low-concentration atropine eye drop’, ‘Atropine’, ‘Eye Drop’, ‘Myopia’, and their Mesh. All studies included were available in English and full-text format. Myopia progression rates were analyzed from all studies, and Rayyan, an online-based tool was used in the screening process.
Results: The results showed that 3 randomized control trials (RCT), 2 cohort studies, and 3 case reports with a total of 1389 participants were analyzed. The majority studies were conducted in Asia, while one RCT was performed in Australia. The participants ranged from 4-12 years old, while atropine eye drop concentrations used were 0.01%, 0.025%, 0.05%, 0.1%, 0.125%, and 0.2%. All studies showed a slower progression rate of myopia in the atropine group compared to the control (-0.31 D vs. -0.90 D; -0.05 D vs. -1.05 D; -0.27 D vs. -0.81 D; -0.28 D vs. -0.54 D; -0.36 D vs. -0.90 D;−0.31 D vs. −0.76 D; -0.31 vs. -0.53 D; -0.38 D vs. -0.55 D) with P < 0.05.
Conclusion: LCA eye drop showed promising effects in slowing myopia progression. However, further investigation is needed, particularly in non-Asian countries.
References
Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-42.
Williams KM, Verhoeven VJ, Cumberland P, Bertelsen G, Wolfram C, Buitendijk GH, et al. Prevalence of refractive error in Europe: the European Eye Epidemiology (E(3)) Consortium. Eur J Epidemiol. 2015;30(4):305-15.
Jeon GS, Hong IH, Lee JH, Song TG, Lee TY, Han JR. Analysis of treatment response about low-dose (0.01%) atropine eye drops in myopic children. Eur J Ophthalmol. 2022;32(4):2011-7.
Kaiti R, Shyangbo R, Sharma IP. Role of Atropine in the Control of Myopia Progression- A Review. Beyoglu Eye J. 2022;7(3):157-66.
Russo A, Boldini A, Romano D, Mazza G, Bignotti S, Morescalchi F, et al. Myopia: Mechanisms and Strategies to Slow Down Its Progression. J Ophthalmol. 2022; 2022:1004977.
Chen CW, Yao JY. Efficacy and Adverse Effects of Atropine for Myopia Control in Children: A Meta-Analysis of Randomised Controlled Trials. J Ophthalmol. 2021; 2021:4274572.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):1-10.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: A revised tool for assessing the risk of bias in randomized trials. BMJ. 2019;366:1-8.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses [Internet]. 2013. Available from: https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp.
Wu PC, Yang YH, Fang PC. The long-term results of using low-concentration atropine eye drops for controlling myopia progression in schoolchildren. J Ocul Pharmacol Ther. 2011;27(5):461-6.
Lee CY, Sun CC, Lin YF, Lin KK. Effects of topical atropine on intraocular pressure and myopia progression: a prospective comparative study. BMC Ophthalmol. 2016;16:114.
Yam JC, Jiang Y, Tang SM, Law AKP, Chan JJ, Wong E, et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmology. 2019;126(1):113-24.
Chuang MN, Fang PC, Wu PC. Stepwise low concentration atropine for myopic control: a 10-year cohort study. Scientific Report. 2021;11:17344.
Jethani J. Efficacy of low-concentration atropine (0.01%) eye drops for prevention of axial myopic progression in premyopes. Indian J Ophthalmol. 2022;70(1):238-40.
Lee SS, Lingham G, Blaszkowska M, Sanfilippo PG, Koay A, Franchina M, et al. Low-concentration atropine eyedrops for myopia control in a multi-racial cohort of Australian children: A randomized clinical trial. Clin Exp Ophthalmol. 2022;50(9):1001-12.
Yam JC, Zhang XJ, Zhang Y, Yip BHK, Tang F, Wong ES, et al. Effect of Low-Concentration Atropine Eyedrops vs Placebo on Myopia Incidence in Children: The LAMP2 Randomized Clinical Trial. JAMA. 2023;329(6):472-81.
Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285-91.
Kaiti R, Shyangbo R, Sharma IP. Role of Atropine in the Control of Myopia Progression- A Review. Beyoglu Eye J. 2022; 7(3):157-66.
Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012;119(2):347-54.
Walline JJ, Berntsen DA. Atropine, 0.01%, for Myopia Control. JAMA Ophthalmol. 2023;141(8):766-7.
Repka MX, Weise KK, Chandler DL, Wu R, Melia BM, Manny RE, et al. Low-Dose 0.01% Atropine Eye Drops vs Placebo for Myopia Control: A Randomized Clinical Trial. JAMA Ophthalmol. 2023;141(8):756-65.
Published
How to Cite
License
Copyright (c) 2023 Siriraj Medical Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














