Efficacy and Safety of Topical 5% Azelaic Acid Solution Versus 2% Minoxidil Solution in the Treatment of Female Pattern Hair Loss
DOI:
https://doi.org/10.33192/smj.v75i12.266001Keywords:
androgenetic alopecia, azelaic acid, female pattern hair loss, minoxidil allergy, pregnancyAbstract
Objective: To determine the efficacy and safety of 5% azelaic acid solution in comparison with 2% minoxidil solution in the treatment of FPHL.
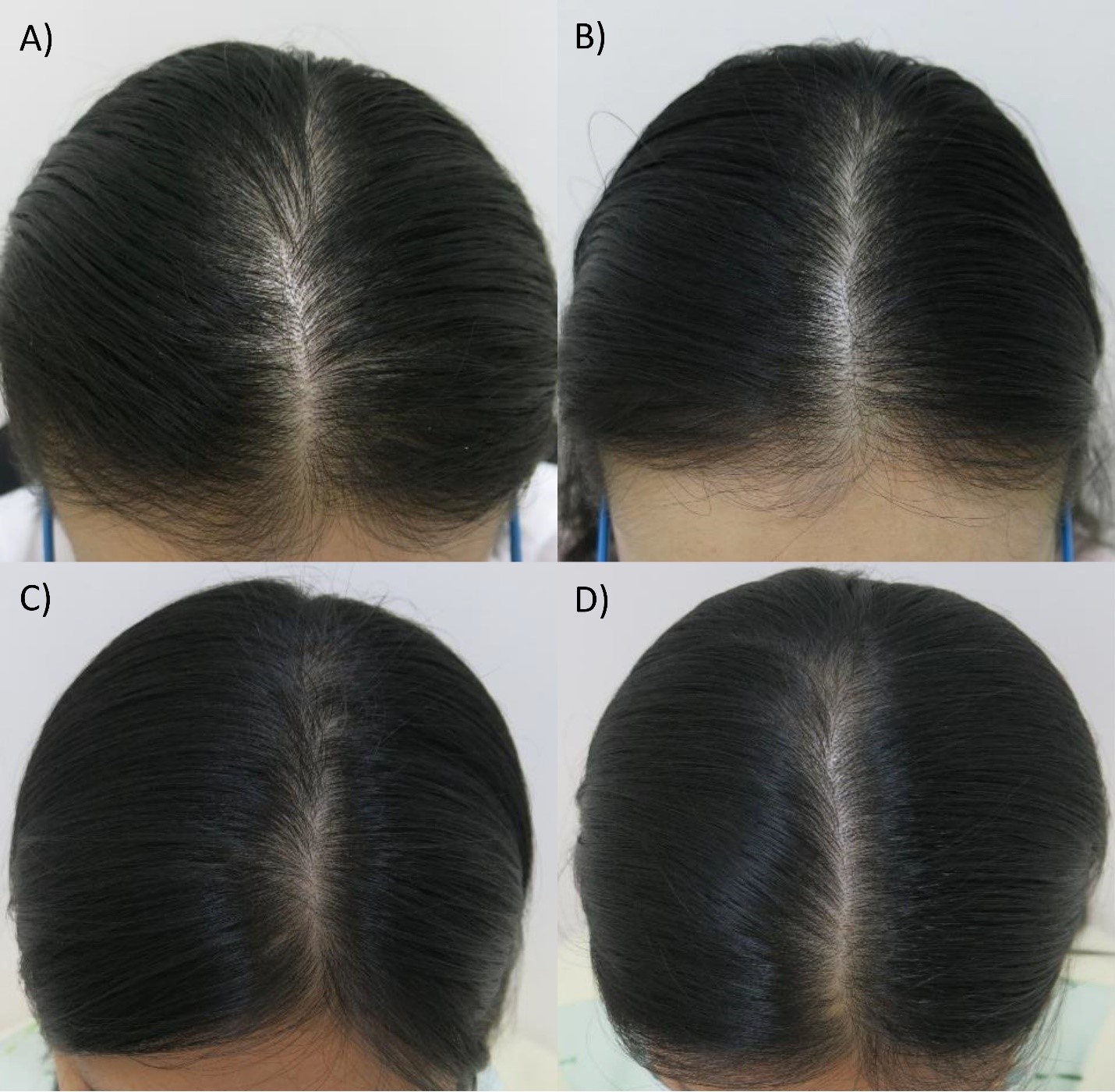
Materials and Methods: Twenty-six FPHL patients with Ludwig grade I or II were randomly treated with 5% azelaic acid solution or 2% minoxidil solution twice daily for 6 months. At baseline, 2, 4, and 6 months, hair density and hair shaft diameter were assessed at the targeted fixed area. At 6 months, patient and investigator assessments of hair growth were performed using a 7-point scale.
Results: Hair density and hair shaft diameter in the patients treated with 5% azelaic acid and 2% minoxidil solution were significantly increased compared to the baseline in all cases and visits (P < 0.05). There were no statistically significant differences in hair density and hair shaft diameter changes between both groups (P > 0.05). Both the investigator and patient assessments were comparable between both groups at 6 months. Pruritus was the major adverse effect reported in both groups, but only mild and all could be tolerated.
Conclusion: 5% Azelaic acid solution might be an effective treatment for FPHL, comparable with 2% minoxidil, and could be an alternative treatment for FPHL in minoxidil-allergic patients and pregnant women.
References
Piraccini BM, Alessandrini A. Androgenetic alopecia. G Ital Dermatol Venereol. 2014;149(1):15-24.
Lolli F, Pallotti F, Rossi A, Fortuna MC, Caro G, Lenzi A, et al. Androgenetic alopecia: a review. Endocrine. 2017;57(1):9-17.
Vujovic A, Del Marmol V. The female pattern hair loss: review of etiopathogenesis and diagnosis. Biomed Res Int. 2014;2014:767628.
Devjani S, Ezemma O, Kelley KJ, Stratton E, Senna M. Androgenetic Alopecia: Therapy Update. Drugs. 2023;83(8):701-15.
Gupta AK, Talukder M, Venkataraman M, Bamimore MA. Minoxidil: a comprehensive review. J Dermatolog Treat. 2022;33(4):1896-906.
Suchonwanit P, Thammarucha S, Leerunyakul K. Minoxidil and its use in hair disorders: a review. Drug Des Devel Ther. 2019;13:2777-86.
Kaler SG, Patrinos ME, Lambert GH, Myers TF, Karlman R, Anderson CL. Hypertrichosis and congenital anomalies associated with maternal use of minoxidil. Pediatrics. 1987;79(3):434-6.
Smorlesi C, Caldarella A, Caramelli L, Di Lollo S, Moroni F. Topically applied minoxidil may cause fetal malformation: a case report. Birth Defects Res A Clin Mol Teratol. 2003;67(12):997-1001.
Trüeb RM, Caballero-Uribe N. Minoxidil-induced hypertrichosis in a breastfed infant. J Eur Acad Dermatol Venereol. 2022;36(3):e224-e5.
Grymowicz M, Rudnicka E, Podfigurna A, Napierala P, Smolarczyk R, Smolarczyk K, et al. Hormonal Effects on Hair Follicles. Int J Mol Sci. 2020;21(15).
Treister-Goltzman Y, Yarza S, Peleg R. Iron Deficiency and Nonscarring Alopecia in Women: Systematic Review and Meta-Analysis. Skin Appendage Disord. 2022;8(2):83-92.
Motosko CC, Bieber AK, Pomeranz MK, Stein JA, Martires KJ. Physiologic changes of pregnancy: A review of the literature. Int J Womens Dermatol. 2017;3(4):219-24.
Searle T, Ali FR, Al-Niaimi F. The versatility of azelaic acid in dermatology. J Dermatolog Treat. 2020:1-11.
Stamatiadis D, Bulteau-Portois MC, Mowszowicz I. Inhibition of 5 alpha-reductase activity in human skin by zinc and azelaic acid. Br J Dermatol. 1988;119(5):627-32.
Amirfakhryan E, Davarnia B, Jeddi F, Najafzadeh N. Azelaic acid stimulates catalase activation and promotes hair growth through upregulation of Gli1 and Gli2 mRNA and Shh protein. Avicenna J Phytomed. 2020;10(5):460-71.
Putra IB, Jusuf NK, Dewi NK. Skin Changes and Safety Profile of Topical Products During Pregnancy. J Clin Aesthet Dermatol. 2022;15(2):49-57.
Pazoki-Toroudi H, Babakoohi S, Nilforoushzadeh MA, Nassiri-Kashani M, Shizarpour M, Ajami M, et al. Therapeutic effects of minoxidil high extra combination therapy in patients with androgenetic alopecia. Skinmed. 2012;10(5):276-82.
Peyravian N, Deo S, Daunert S, Jimenez JJ. The Inflammatory Aspect of Male and Female Pattern Hair Loss. J Inflamm Res. 2020;13:879-81.
Lucky AW, Piacquadio DJ, Ditre CM, Dunlap F, Kantor I, Pandya AG, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50(4):541-53.
McGowan MA, Scheman A, Jacob SE. Propylene Glycol in Contact Dermatitis: A Systematic Review. Dermatitis. 2018;29(1):6-12.
Blume-Peytavi U, Hillmann K, Dietz E, Canfield D, Garcia Bartels N. A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. J Am Acad Dermatol. 2011;65(6):1126-34.e2.
Published
How to Cite
License
Copyright (c) 2023 Siriraj Medical Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














