Nicotine Gum in Thai Smokers with Different CYP2A6 Enzymes: A Population Pharmacokinetic Analysis
DOI:
https://doi.org/10.33192/smj.v76i8.267636Keywords:
population pharmacokinetics, nicotine chewing gum, Cytochrome P-450 CYP2A6Abstract
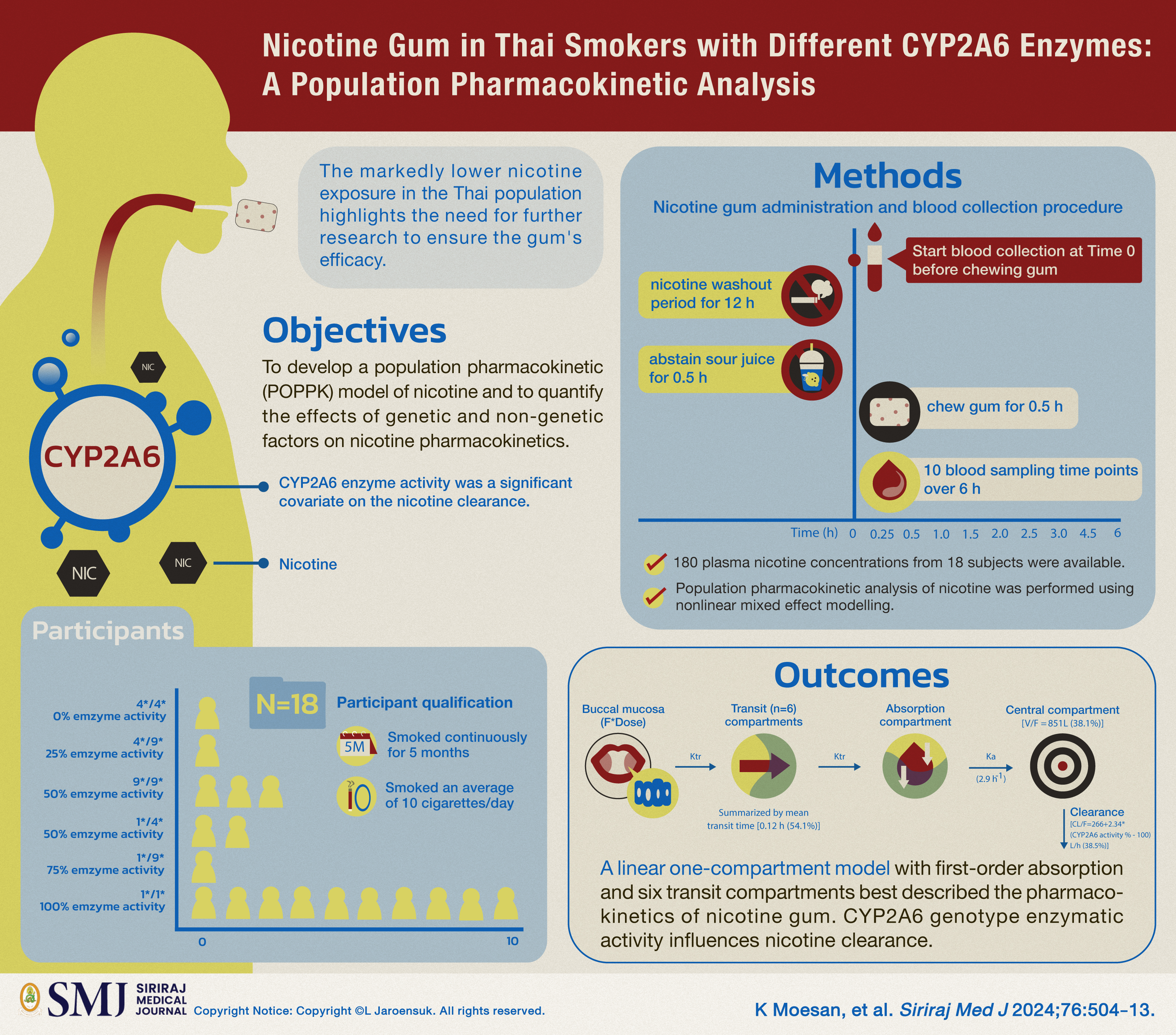
Objective: Despite the popularity of nicotine gum in Thailand, population pharmacokinetics of nicotine gum in the Thai population has not been investigated. This study aimed to develop a population pharmacokinetic (POPPK) model of nicotine and to quantify the effects of genetic and non-genetic factors to nicotine pharmacokinetics.
Materials and Methods: Secondary data collected from a previous clinical trial assessing cytochrome P450 2A6 (CYP2A6) genotypes in Thai smokers was investigated. Eighteen participants who had received a single dose of 2 mg nicotine gum were included. Blood samples were collected before, at 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4.5 and 6 hours after nicotine administration. POPPK analysis was performed using nonlinear mixed effect modelling.
Results: One-compartment with 1st order elimination and absorption with 6-transit compartments best described the data. CYP2A6 enzyme activity was a significant covariate on the nicotine clearance. Apparent elimination clearance (CL/F) for a person with 100% CYP2A6 activity was 266.0 L/h. CL/F would be 36.0 L/h in a subject with 0% CYP2A6 activity. However, the impact of non-genetic factors (monthly alcohol consumption, Fagerstrom Test for Nicotine Dependence score and the number of cigarettes per day) on pharmacokinetics of nicotine were not found.
Conclusion: This first report on population pharmacokinetics of nicotine gum in Thai smokers provided the pharmacokinetic model and quantified CL/F for smokers with different CYP2A6 genotypes. A markedly lower exposure to nicotine in the Thai population compared to others highlights the need for more studies to ensure the efficacy of nicotine gum in the Thai population.
References
World Health Organization. Heart disease and stroke are one of the commonest ways by which tobacco kills people. Available from: http://www.who.int/iris/bitstream/10665/272690/1/wntd_2018_thailand_fs.pdf. (Accessed: 17 July, 2019).
Zhao J, Pachanee CA, Yiengprugsawan V, Seubsman SA, Sleigh A. Smoking, smoking cessation, and 7-year mortality in a cohort of Thai adults. Popul Health Metr. 2015 Dec;13(1):1-0.
Chaikoolvatana A, Pheunpha P, Puchcharanapaponthorn P, Chaikoolvatana C, Saisingh N, Suwannakoot P, et al. The Evaluation of Initiating Tobacco Cessation Services in the Military-Based Hospital, Northeastern Thailand. Siriraj Med J. 2015;67(4):160-7.
Hartmann‐Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev. 2018;5(5):CD000146.
Chinwong S, Chinwong D. A national survey of community pharmacists on smoking cessation services in Thailand. Pharmacy (Basel). 2018;6(3):101.
Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79-115.
Benowitz NL, Jacob III P, Savanapridi C. Determinants of nicotine intake while chewing nicotine polacrilex gum. Clin Pharmacol Ther. 1987;41(4):467-73.
McNabb ME, Ebert RV, McCusker K. Plasma nicotine levels produced by chewing nicotine gum. JAMA. 1982;248(7):865-8.
Brossard P, Weitkunat R, Poux V, Lama N, Haziza C, Picavet P, Baker G, Lüdicke F. Nicotine pharmacokinetic profiles of the Tobacco Heating System 2.2, cigarettes and nicotine gum in Japanese smokers. Regul Toxicol Pharmacol. 2017;89:193-9.
Choi JH, Dresler CM, Norton MR, Strahs KR. Pharmacokinetics of a nicotine polacrilex lozenge. Nicotine Tob Res. 2003;5(5):635-44.
Dautzenberg B, Nides M, Kienzler JL, Callens A. Pharmacokinetics, safety and efficacy from randomized controlled trials of 1 and 2 mg nicotine bitartrate lozenges (Nicotinell®). BMC Clin Pharmacol. 2007;7:1-5.
Du D. A single-dose, crossover-design bioequivalence study comparing two nicotine gum formulations in healthy subjects. Adv Ther. 2018;35:1169-80.
Garg M, Naidu R, Iyer K, Jadhav R. Bioequivalence of two different nicotine chewing gum formulations of two different strengths (2 mg and 4 mg) in Indian healthy adult human male smoker subjects. J Bioequiv Availab. 2016;8:074-9.
Hansson A, Rasmussen T, Kraiczi H. Single-dose and multiple-dose pharmacokinetics of nicotine 6 mg gum. Nicotine Tob Res. 2017;19(4):477-83.
Chen LS, Bloom AJ, Baker TB, et al. Pharmacotherapy effects on smoking cessation vary with nicotine metabolism gene (CYP2A6). Addiction. 2014;109(1):128-37.
Tanner JA, Tyndale RF. Variation in CYP2A6 activity and personalized medicine. J Pers Med. 2017;7(4):18.
Marchand M, Brossard P, Merdjan H, Lama N, Weitkunat R, Lüdicke F. Nicotine population pharmacokinetics in healthy adult smokers: a retrospective analysis. Eur J Drug Metab Pharmacokinet. 2017;42(6):943-54.
Olsson Gisleskog PO, Perez Ruixo JJ, Westin Å, Hansson AC, Soons PA. Nicotine population pharmacokinetics in healthy smokers after intravenous, oral, buccal and transdermal administration. Clin Pharmacokinet. 2021;60:541-61.
Beal SL, Sheiner LB, Boeckmann AJ, Bauer RJ. NONMEM Users Guides. 1989-2011. Icon Development Solutions, Ellicott City, Maryland, USA. 2011;1(1):1.
Jonsson EN, Karlsson MO. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58(1):51-64.
Kumondai M, Hosono H, Orikasa K, Arai Y, Arai T, Sugimura H, et al. Genetic polymorphisms of CYP2A6 in a case-control study on bladder cancer in Japanese smokers. Biol Pharm Bull. 2016;39(1):84-9.
Nguyen TH, Mouksassi MS, Holford N, Al-Huniti N, Freedman I, Hooker AC, et al. Model Evaluation of Continuous Data Pharmacometric Models: Metrics and Graphics. CPT Pharmacometrics Syst Pharmacol. 2017;6(2);87-109.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. Aaps J. 2011;13(2): 143-51.
Dempsey D, Tutka P, Jacob P 3rd, Allen F, Schoedel K, Tyndale RF, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76(1):64-72.
Hicks JK, Sangkuhl K, Swen JJ, Ellingrod VL, Muller DJ, Shimoda K, et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharmacol Ther. 2017;102(1):37-44.
Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther. 2008;83(2):234-42.
Dermody SS, Hendershot CS, Andrade AK, Novalen M, Tyndale RF. Changes in nicotine metabolite ratio among daily smokers receiving treatment for alcohol use disorder. Nicotine Tob Res. 2020;22(2):256-63.
Gubner NR, Kozar-Konieczna A, Szoltysek-Boldys I, Slodszyk-Mankowska E, Goniewicz J, Sobczak A, et al. Cessation of alcohol consumption decreases rate of nicotine metabolism in male alcohol-dependent smokers. Drug Alcohol Depend. 2016;163:157-64.
Dansirikul C, Silber HE, Karlsson MO. Approaches to handling pharmacodynamic baseline responses. J Pharmacokinet Pharmacodyn. 2008;35(3):269-83.
Chinwong D, Mookmanee N, Chongpornchai J, Chinwong S. A comparison of gender differences in smoking behaviors, intention to quit, and nicotine dependence among Thai university students. J Addict. 2018;2018:8081670.
Published
How to Cite
License
Copyright (c) 2024 Siriraj Medical Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














