Cancer Immunotherapy: Challenges and Advancements in CAR T Cell Technology
DOI:
https://doi.org/10.33192/smj.v76i5.268031Keywords:
Cancer immunotherapy, Adoptive T-cell transfer, Chimeric antigen receptor (CAR) T cells, Fourth-generation and fifth-generation CAR T cells, Solid tumor applicationAbstract
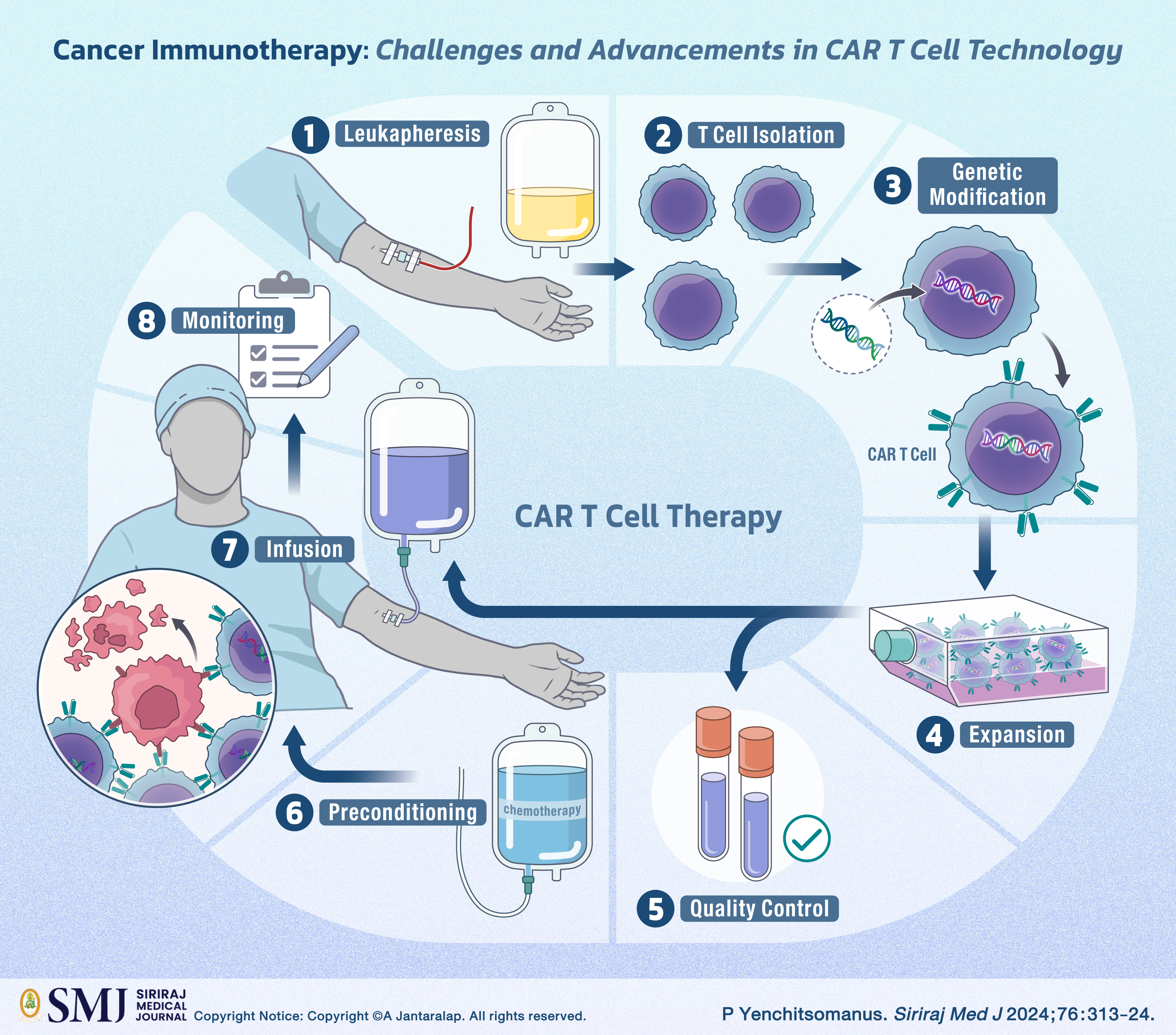
Cancer, characterized by uncontrolled cell proliferation, poses a major global health threat, as evident in the 2022 World Health Organization-International Agency for Research on Cancer report, recording 20 million new cases and 9.7 million deaths worldwide. Thailand alone reported 183,000 new cases and 118,000 fatalities, underscoring the need for tailored prevention, early detection, and treatment strategies. Conventional therapies like surgery, radiation, and chemotherapy, while effective in early stages, face limitations in advanced cases, prompting the development of targeted therapies and immunotherapy, notably chimeric antigen receptor (CAR) T cell therapy. CAR T cell therapy employs genetic engineering to create receptors recognizing cancer-specific antigens. Despite successes in hematological malignancies, challenges such as toxicities, relapse, and high costs persist. Ongoing research, led by the Siriraj Center of Research Excellence for Cancer Immunotherapy (SiCORE-CIT), focuses on advancing fourth- and fifth-generation CAR T cell technologies. SiCORE-CIT's fourth-generation CAR T cells exhibit potent anti-tumor activity against various cancers, surpassing second-generation counterparts. Their innovative fifth-generation "Siriraj fifth-generation CAR T cells" secrete anti-PD-L1 scFv, showing potential for diverse cancer applications, highlighting the transformative impact of ongoing research. Successful applications of fifth-generation CAR T cells in B-cell leukemia, lymphoma, and multiple myeloma underscore their transformative potential. This emphasizes the critical role of continuous research in refining therapeutic approaches for both hematologic and solid malignancies. The ongoing exploration and development in this domain have the potential to revolutionize cancer treatment paradigms, significantly contributing to alleviating the global health burden associated with this complex disease.
References
The International Agency for Research on Cancer (IARC), World Health Organization (WHO). Press release no. 345: Global cancer burden growing, amidst mounting need for services. 1 February 2024.
The International Agency for Research on Cancer (IARC), World Health Organization (WHO), Global Cancer Observatory, Cancer Today, GLOBOCAN 2022, Population Factsheets: Thailand.
Chen S, Cao Z, Prettner K, Kuhn M, Yang J, Jiao L, Wang Z, Li W, Geldsetzer P, Bärnighausen T, Bloom DE, Wang C. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. 2023;9(4):465-72.
Editorial. Advancing Cancer Therapy. Nat Cancer. 2021;2(3):245-246.
June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med 2018;379(1):64-73.
Boyiadzis MM, Dhodapkar MV, Brentjens RJ, Kochenderfer JN, Neelapu SS, Maus MV, et al. Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: clinical perspective and significance. J Immunother Cancer. 2018;6:137.
June CH. Q&A: Carl June on CAR T-cell Therapy. Blood Cancer Discov 2020;1(1):8–9.
Newitt VN. The incredible story of Emily Whitehead & CAR T-cell therapy. Oncology Times 2022;44(6):1,19-20.
Prasad V. Immunotherapy: Tisagenlecleucel - the first approved CAR-T-cell therapy: implications for payers and policy makers. Nat Rev Clin Oncol. 2018;15(1):11-12.
Cappell KM Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. 2023;20(6):359-71.
Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naïve or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20-28.
Dai Z, Mu W, Zhao Y, Cheng J, Lin H, Ouyang K et al. T cells expressing CD5/CD7 bispecific chimeric antigen receptors with fully human heavy-chain-only domains mitigate tumor antigen escape. Signal Transduct Target Ther. 2022;7(1):85.
Can I, Cox MJ, Siegler EL, Sakemura R, Kenderian SS. Challenges of chimeric antigen receptor T-cell therapy in chronic lymphocytic leukemia: lessons learned. Exp Hematol. 2022:108:1-7.
Turkalj S, Radtke FA, Vyas P. An overview of targeted therapies in acute myeloid leukemia. Hemaspere 2023;7(6):e914.
Susanibar-Adaniya S, Barta SK. 2021 Update on Diffuse large B cell lymphoma: A review of current data and potential applications on risk stratification and management. J Hematol. 2021;96(5):617-29.
Schultz L, Mackall C. Driving CAR T cell translation forward. Sci Transl Med. 2019;11(481):eaaw2127.
Hasanali ZS, Razzo B, Susanibar-Adaniya SP, Garfall AL, Stadtmauer EA, Cohen AD. Chimeric antigen receptor T cells in the treatment of multiple myeloma. Hematol Oncol Clin North Am. 2023 Dec 28:S0889-8588(23)00171-5.
Braunstein M, Weltz J, Davies F. A new decade: novel immunotherapies on the horizon for relapsed/refractory multiple myeloma. Expert Rev Hematol. 2021;14(4):377-89.
Chohan KL, Siegler EL, Kenderian SS. CAR-T cell therapy: the efficacy and toxicity balance. Curr Hematol Malig Rep. 2023;18(2):9-18.
Dourthe M-E, Rabian F, Yakouben K, Chevillon F, Cabannes-Hamy A, Méchinaud F, et al. Determinants of CD19-positive vs CD19-negative relapse after tisagenlecleucel for B-cell acute lymphoblastic leukemia. Leukemia. 2021;35(12):3383-93.
Hay AE, Cheung MC. CAR T-cells: costs, comparisons, and commentary. J Med Econ. 2019;22(7):613-5.
National Cancer Institute. CAR T cells: engineering patients’ immune cells to treat their cancers. https://www.cancer.gov/about-cancer/treatment/research/car-t-cells.
Simsek H, Klotzsch E. The solid tumor microenvironment-breaking the barrier for T cells: how the solid tumor microenvironment influences T cells. Bioessays. 2022;44(6):e2100285.
Albelda SM. CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn. Nat Rev Clin Oncol. 2024;21(1):47-66.
Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3(12):939-51.
Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. CD28 costimulation; from mechanism to therapy. Immunity 2016;44(5):973-88.
Otano I, Azpilikueta A, Glez-Vaz J, Alvarez M, Medina-Echeverz J, Cortés-Domínguez I, et al. CD137 (4-1BB) costimulation of CD8+ T cells is more potent when provided in cis than in trans with respect to CD3-TCR stimulation. Nat Commun. 2021;12(1):7296.
Starzer AM, Berghoff AS. New emerging targets in cancer immunotherapy: CD27 (TNFRSF7). ESMO Open. 2020;4(Suppl 3):e000629.
Wutti-In Y, Sujjitjoon J, Sawasdee N, Panya A, Kongkla K, Yuti P, et al. Development of a novel anti-CD19 CAR containing a fully human scFv and three costimulatory domains. Front Oncol. 2022;11:802876.
Sangsuwannukul T, Supimon K, Sujjitjoon J, Phanthaphol N, Chieochansin T, Poungvarin N, et al. Anti-tumour effect of the fourth-generation chimeric antigen receptor T cells targeting CD133 against cholangiocarcinoma cells. Int Immunopharmacol. 2020;89(Pt B):107069.
Supimon K, Sangsuwannukul T, Sujjitjoon J, Phanthaphol N, Chieochansin T, Poungvarin N, et al. Anti-mucin 1 chimeric antigen receptor T cells for adoptive T cell therapy of cholangiocarcinoma. Sci Rep. 2021;11(1):6276.
Phanthaphol N, Somboonpatarakun C, Suwanchiwasiri K, Chieochansin T, Sujjitjoon J, Wongkham S, et al. Chimeric antigen receptor T cells targeting integrin αvβ6 expressed on cholangiocarcinoma cells. Front Oncol. 2021;11:657868.
Luangwattananun P, Junking M, Sujjitjoon J, Wutti-In Y, Poungvarin N, Thuwajit C, et al. Fourth-generation chimeric antigen receptor T cells targeting folate receptor alpha antigen expressed on breast cancer cells for adoptive T cell therapy. Breast Cancer Res Treat. 2021;186(1):25-36.
Somboonpatarakun C, Phanthaphol N, Suwanchiwasiri K, Ramwarungkura B, Yuti P, Poungvarin N, Thuwajit P, et al. Cytotoxicity of fourth-generation anti-Trop2 CAR-T cells against breast cancer. Int Immunopharmacol. 2024;129:111631.
Yuti P, Wutti-In Y, Sawasdee N, Kongkhla K, Phanthaphol N, Choomee K, et al. Anti-CD19 chimeric antigen receptor T cells secreting anti-PD-L1 single-chain variable fragment attenuate PD-L1 mediated T cell inhibition. Int Immunopharmacol. 2022;113(Pt B):109442.
Yuti P, Sawasdee N, Natungnuy K, Rujirachaivej P, Luangwattananun P, Sujjitjoon J, et al. Enhanced antitumor efficacy, proliferative capacity, and alleviation of T cell exhaustion by fifth-generation chimeric antigen receptor T cells targeting B cell maturation antigen in multiple myeloma. Biomed Pharmacother. 2023;168:115691.
Published
How to Cite
License
Copyright (c) 2024 Siriraj Medical Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














