Optimization of the Use of the DOTATATE Kit Manufactured by the Thailand Institute of Nuclear Technology Using a SiO2-based 68Ge/68Ga Generator
DOI:
https://doi.org/10.33192/smj.v76i11.270157Keywords:
68Ga-DOTATATE, TINT kit, Neuroendocrine tumor, Ga-68 elution, Radiochemical purityAbstract
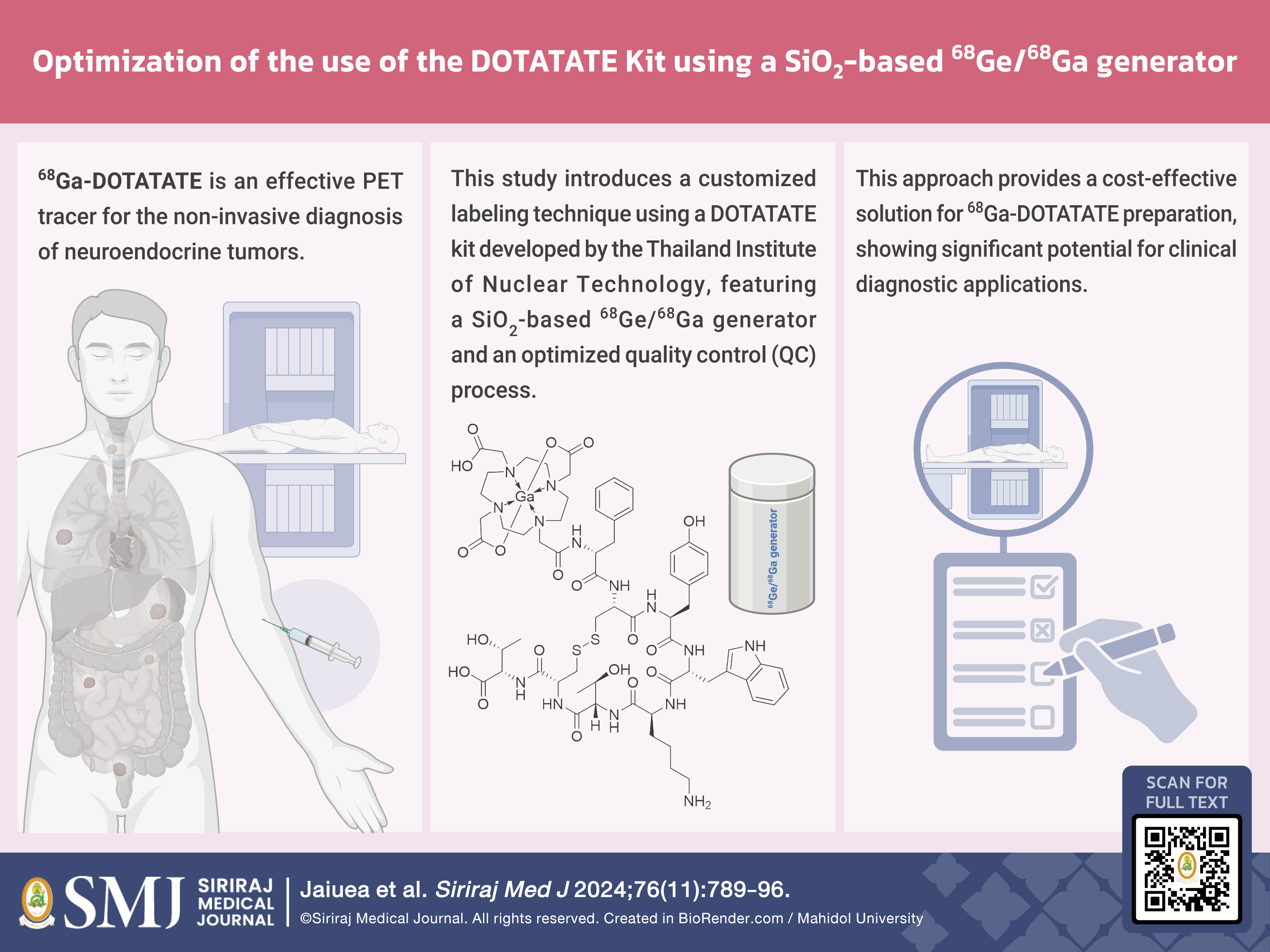
Objective: The presence of somatostatin receptors on neuroendocrine tumours enables 68Ga-DOTATATE to precisely detect lesion localization and staging. Thailand Institute of Nuclear Technology (TINT) recently developed a DOTATATE kit for labelling with Ga-68, which is compatible with a TiO2-based 68Ge/68Ga generator eluted with 0.1 M HCl, but presents a discrepancy with other types of 68Ge/68Ga generators. This research aimed to optimize a radiolabelling method using TINT’s kit with a SiO2-based 68Ge/68Ga generator eluting Ga-68 in 0.05 M HCl. Additionally, a quality control protocol was developed to ensure the formulation’s efficacy and reliability in compliance with the 10th edition of the European Pharmacopoeia.
Material and Methods: The SiO2-based 68Ge/68Ga generator was eluted with 2–4 ml of 0.05 M HCl, added into a lyophilized kit, heated in a dried-block heater at 100 ºC for 15 min, cooled down at room temperature, and finally purified using Sep-Pak C18 cartridge. The radiochemical purity was determined by radio thin-layer chromatography and the radioactivity was measured by a gamma well counter. Reproducibility and stability tests were conducted three times.
Results: Employing 4 ml of eluted material, comprising the second and fifth millilitres of 68GaCl3, provided a radiochemical purity (RCP) exceeding 95% after purification. Also, 68Ga-DOTATATE remained stable in refrigerator for at least 4 half-lives.
Conclusion: TINT’s DOTATATE kit can be successfully labelled with a SiO2-based 68Ge/68Ga generator, providing 68Ga-DOTATATE with an RCP > 95% for at least 4 half-lives when stored in refrigerator after production. This radiolabelling procedure is suitable for routine clinical application.
References
Ichikawa Y, Kobayashi N, Takano S, Kato I, Endo K, Inoue T. Neuroendocrine tumor theranostics. Cancer Sci. 2022;113(6):1930-8.
Klomp M, Dalm S, De Jong M, Feelders R, Hofland J, Hofland L. Epigenetic regulation of somatostatin and somatostatin receptors in neuroendocrine tumors and other types of cancer. Rev Endocr Metab Disord. 2021;22(3):495-510.
Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335-42.
Papotti M, Bongiovanni M, Volante M, Allia E, Landolfi S, Helboe L, et al. Expression of somatostatin receptor types 1–5 in 81 cases of gastrointestinal and pancreatic endocrine tumors: a correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch. 2002;440(5):461-75.
Olias G, Viollet C, Kusserow H, Epelbaum J, Meyerhof W. Regulation and function of somatostatin receptors. J Neurochem. 2004;89(5):1057-91.
Barnett P. Somatostatin and somatostatin receptor physiology. Endocrine. 2003;20(3):255-64.
Gatto F, Barbieri F, Arvigo M, Thellung S, Amarù J, Albertelli M, et al. Biological and biochemical basis of the differential efficacy of first and second generation somatostatin receptor ligands in neuroendocrine neoplasms. Int J Mol Sci. 2019;20(16):3940.
Barbieri F, Bajetto A, Pattarozzi A, Gatti M, Würth R, Thellung S, et al. Peptide receptor targeting in cancer: the somatostatin paradigm. Int J Pept. 2013;2013:926295.
Battershill PE, Clissold SP. Octreotide: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in conditions associated with excessive peptide secretion. Drugs. 1989;38(5):658-702.
Sanli Y, Garg I, Kandathil A, Kendi T, Zanetti MJB, Kuyumcu S, et al. Neuroendocrine tumor diagnosis and management: 68Ga-DOTATATE PET/CT. AJR Am J Roentgenol. 2018;211(2):267-77.
Capdevila J, Sevilla I, Alonso V, Antón Aparicio L, Jiménez Fonseca P, Grande E, et al. Evaluation of the efficacy and safety of lanreotide in combination with targeted therapies in patients with neuroendocrine tumours in clinical practice: a retrospective cross-sectional analysis. BMC Cancer. 2015;15:495.
Calès P. Vapreotide acetate for the treatment of esophageal variceal bleeding. Expert Rev Gastroenterol Hepatol. 2008;2(2):185-92.
Kubicek V, Havlickova J, Kotek J, Tircso G, Hermann P, Toth E, et al. Gallium (III) complexes of DOTA and DOTA− monoamide: kinetic and thermodynamic studies. Inorg Chem. 2010;49(23):10960-9.
Stueven AK, Kayser A, Wetz C, Amthauer H, Wree A, Tacke F, et al. Somatostatin analogues in the treatment of neuroendocrine tumors: past, present and future. Int J Mol Sci. 2019;20(12):3049.
Bergsma H, van Vliet EI, Teunissen JJ, Kam BL, de Herder WW, Peeters RP, et al. Peptide receptor radionuclide therapy (PRRT) for GEP-NETs. Best Pract Res Clin Gastroenterol. 2012;26(6):867-81.
Van Essen M, Krenning EP, De Jong M, Valkema R, Kwekkeboom DJ. Peptide receptor radionuclide therapy with radiolabelled somatostatin analogues in patients with somatostatin receptor positive tumours. Acta Oncologica. 2007;46(6):723-34.
U.S. Food and Drug Administration. FDA approves new diagnostic imaging agent to detect rare neuroendocrine tumors 2016. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-diagnostic-imaging-agent-detect-rare-neuroendocrine-tumors.
Mukherjee A, Pandey U, Chakravarty R, Sarma HD, Dash A. Development of single vial kits for preparation of 68Ga-labelled peptides for PET imaging of neuroendocrine tumours. Mol Imaging Biol. 2014;16(4):550-7.
Mukherjee A, Pandey U, Chakravarty R, Sarma HD, Dash A. Single vial kit formulation for preparation of PET radiopharmaceutical: 68Ga-DOTA-TOC. Journal of Radioanalytical and Nuclear Chemistry. 2014;302:1253-8.
Das T, Bhadwal M, Sarma H, Banerjee S. Formulation and radiochemical evaluation of a freeze-dried mixed peptide kit for the preparation of 68Ga-labeled peptides for PET imaging of somatostatin receptor positive neuroendocrine cancers. Journal of Radioanalytical and Nuclear Chemistry. 2014;302:1259-64.
Asti M, Iori M, Capponi PC, Rubagotti S, Fraternali A, Versari A. Development of a simple kit-based method for preparation of pharmaceutical-grade 68Ga-DOTATOC. Nucl Med Commun. 2015;36(5):502-10.
Mukherjee A, Korde A, Sarma HD, Samuel G. Single vial formulation for theranostic radiopharmaceutical preparation. Journal of Radioanalytical and Nuclear Chemistry. 2014;302:889-94.
Prince D, Rossouw D, Davids C, Rubow S. Development and evaluation of user-friendly single vial DOTA-peptide kit formulations, specifically designed for radiolabelling with 68Ga from a tin dioxide 68Ge/68Ga generator. Mol Imaging Biol. 2017;19(6):817-24.
Sriprapa T, Doungta T, Sakulsamart N, Taweewatthanasopon N, Madputeh L, Ragchana P, et al. Evaluation of the Efficacy and Safety of the ITM 68Ge/68Ga Generator After its Recommended Shelf-life. Siriraj Med J. 2023;75(10):752-8.
Breeman WA, de Jong M, de Blois E, Bernard BF, Konijnenberg M, Krenning EP. Radiolabelling DOTA-peptides with 68Ga. Eur J Nucl Med Mol Imaging. 2005;32(4):478-85.
Velikyan I, Beyer GJ, Långström B. Microwave-supported preparation of 68Ga bioconjugates with high specific radioactivity. Bioconjug Chem. 2004;15(3):554-60.
European Pharmacopeia. Gallium (68Ga) Edotreotide injection monograph. In: European Pharmacopeia (EP). 10th editor. European Pharmacopeia; 2019.p.1208-10.
Published
How to Cite
License
Copyright (c) 2024 Siriraj Medical Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














