Minimum Ischiopubic Ramus Width as a Single Metric for Sex Estimation: A Pilot Study
DOI:
https://doi.org/10.33192/smj.v77i12.277804Keywords:
Ischiopubic ramus, sex estimation, fragmented bones, machine learningAbstract
Objective: The present study assesses the effectiveness of the minimum width of the ischiopubic ramus as a single measurement for sex estimation.
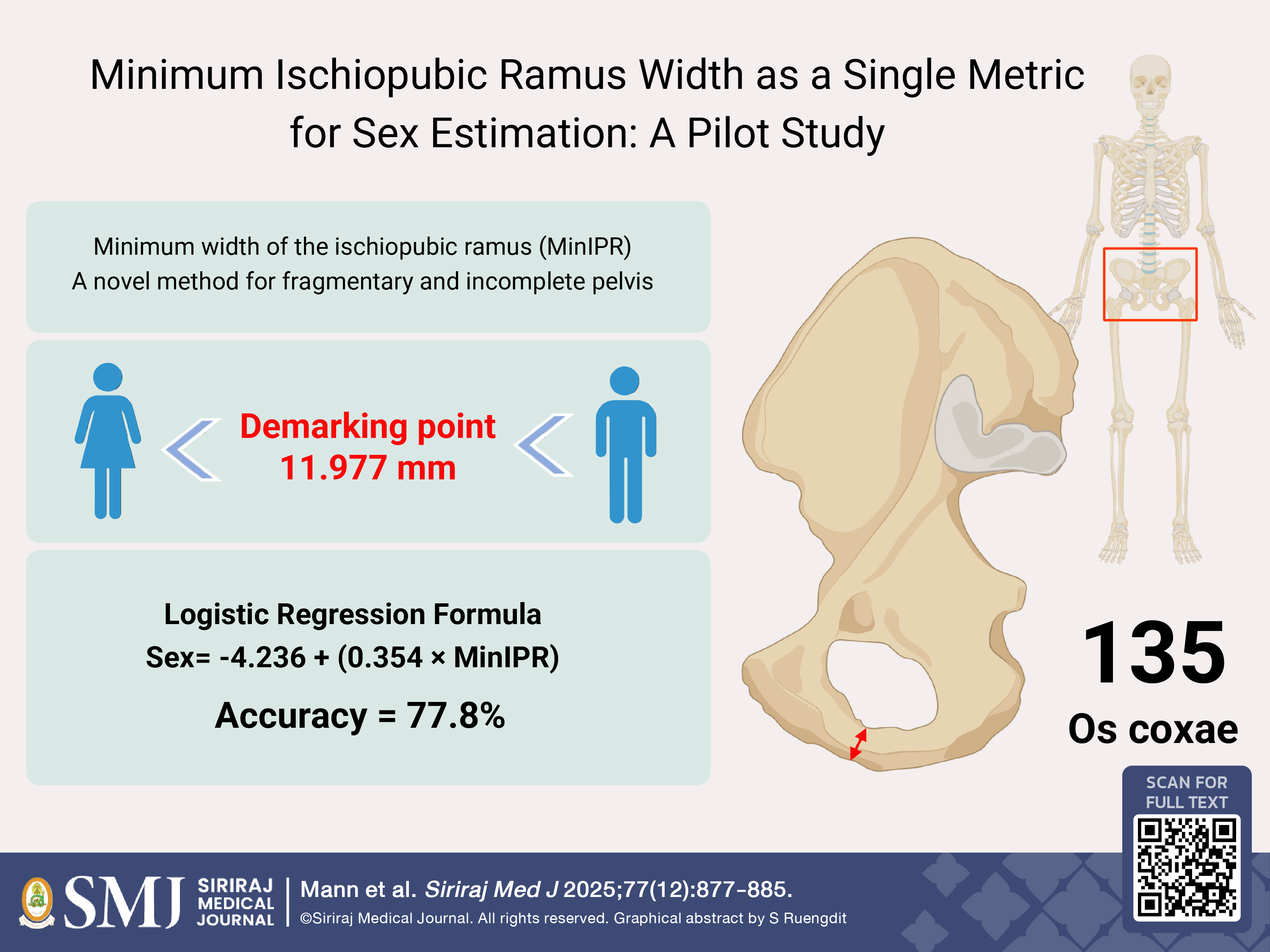
Materials and Methods: Visual and metrical examination of 135 known-identity male and female os coxae was investigated by measuring the minimum mediolateral width of the ischiopubic ramus, a novel and simple measurement of the narrowest part of the ischiopubic ramus.
Results: The results revealed females typically have a narrow (“pinched”) ischiopubic ramus in comparison to males, with a statistically significant difference in the minimum mediolateral width of the ischiopubic ramus between sexes. Logistic regression, which delivered the best sex estimation model among traditional statistical analysis and machine learning approaches, provided an accuracy of 77.8%, a sensitivity of 83.33% and a specificity of 73.33% with a demarking point of 11.977 mm.
Conclusion: Although the method yields moderate accuracy, the minimum width of the ischiopubic ramus provides a straightforward and practical approach that may assist in sex estimation when only a fragment of the ischiopubic ramus is available. Further validation using larger and more diverse samples is recommended to confirm its applicability.
References
Klales AR. Sex estimation using pelvis morphology. In: Klales AR, ed. Sex estimation of the human skeleton: history, methods, and emerging techniques, Cambridge: Academic Press, 2020. p. 75-93.
Buikstra JE, Ubelaker DH. Standards for Data Collection from Human Skeletal Remains, Fayetteville: 1994.
Krishan K, Chatterjee PM, Kanchan T, Kaur S, Baryah N, Singh RK. A review of sex estimation techniques during examination of skeletal remains in forensic anthropology casework. Forensic Sci Int. 2016;261:165.e1-.e8.
Black V. Sex estimation using geometric morphometrics: evaluation of elements of the Pubis. Forensic Anthropology. 2021;4(3):47.
Waldron T. A report on the human bone from Merton Priory: English Heritage; 1985.
Mann RW, Koel-Abt K, Dhody A, Mahakkanukrauh P, Mann VJ, Techataweewan N, et al. The importance of human osteological collections: Our past, present, and future. Forensic Sci Int. 2021;325:110895.
Chomean S, Chatthai N, Sangchay N, Kaset C. Enhancing forensic sex identification through AI-based analysis of the foramen magnum. Forensic Science International: Reports. 2025;11:100411.
R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2025. Available from: https://www.R-project.org/
Harris SM, Case DT. Sexual dimorphism in the tarsal bones: implications for sex determination. J Forensic Sci. 2012;57(2):295-305.
Knapp TR. Technical error of measurement: a methodological critique. American Journal of Physical Anthropology. 1992;87(2):235-6.
Ward R, Jamison P. Measurement precision and reliability in craniofacial anthropometry: implications and suggestions for clinical applications. J Craniofac Genet Dev Biol. 1991;11(3):156-64.
Lovell NC. Test of Phenice's technique for determining sex from the os pubis. Am J Phys Anthropol. 1989;79(1):117-20.
Suchey JM, Sutherland LD. Use of the ventral arc in pubic sex determination. J Forensic Sci. 1991;36(2):501-11.
Ubelaker DH, Volk CG. A test of the phenice method for the estimation of sex. J Forensic Sci. 2002;47(1):19-24.
Luo Y-C. Sex determination from the pubis by discriminant function analysis. Forensic Sci Int. 1995;74(1):89-98.
Dixit S, Kakar S, Agarwal S, Choudhry R. Sexing of human hip bones of Indian origin by discriminant function analysis. J Forensic Leg Med. 2007;14(7):429-35.
Blake KA, Hartnett‐McCann K. Metric assessment of the pubic bone using known and novel data points for sex estimation. J Forensic Sci. 2018;63(5):1472-8.
Patriquin M, Steyn M, Loth S. Metric analysis of sex differences in South African black and white pelves. Forensic Sci Int. 2005;147(2-3):119-27.
Bytheway JA, Ross AH. A geometric morphometric approach to sex determination of the human adult os coxa. J Forensic Sci. 2010;55(4):859-64.
Gonzalez PN, Bernal V, Perez SI. Geometric morphometric approach to sex estimation of human pelvis. Forensic Sci Int. 2009;189(1-3):68-74.
Torimitsu S, Makino Y, Saitoh H, Sakuma A, Ishii N, Yajima D, et al. Morphometric analysis of sex differences in contemporary Japanese pelves using multidetector computed tomography. Forensic Sci Int. 2015;257:530.e1-e7.
Mahakkanukrauh P, Ruengdit S, Tun SM, Case DT, Sinthubua A. Osteometric sex estimation from the os coxa in a Thai population. Forensic Sci Int. 2017;271:127.e1-e7.
Vacca E, Di Vella G. Metric characterization of the human coxal bone on a recent Italian sample and multivariate discriminant analysis to determine sex. Forensic Sci Int. 2012;222(1–3):401.e1-e9.
Novotný V. Sex determination of the pelvic bone: a system approach. Anthropologie Paris. 1986;24(2-3):197-206.
Steyn M, İşcan M. Metric sex determination from the pelvis in modern Greeks. Forensic Sci Int. 2008;179(1):86.e1-e6.
Franklin D, Cardini A, Flavel A, Marks MK. Morphometric analysis of pelvic sexual dimorphism in a contemporary Western Australian population. Int J Legal Med. 2014;128(5):861-72.
Arun M, Nagesh K, Kumar GP. Estimation of sex from fragments of os coxa by metric analysis. Australian Journal of Forensic Sciences. 2012;44(2):145-53.
Sangchay N, Dzetkuličová V, Zuppello M, Chetsawang J. Consideration of accuracy and observational error analysis in pelvic sex assessment: A study in a Thai cadaveric human population. Siriraj Med J. 2022;74(5):330-339.
Lüthje P, Nurmi N, Kataja M, Heliövaara M, Santavirta S. Incidence of pelvic fractures in Finland in 1988. Acta Orthop Scand. 1995;66(3):245-8.
Ragnarsson B, Jacobsson B. Epidemiology of pelvic fractures in a Swedish county. Acta Orthop Scand. 1992;63(3):297-300.
Kane WJ. Fracture of the pelvis. In: Rockwood CA, Green DP, eds. Fractures in Adults, Philadelphia: J.B. Lippincott, 1984.p.1093-209.
Rogers T, Saunders S. Accuracy of sex determination using morphological traits of the human pelvis. J Forensic Sci. 1994;39(4):1047-56.
Published
How to Cite
Issue
Section
Categories
License
Copyright (c) 2025 Siriraj Medical Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














