In Silico Study of Functional Network Analysis Focusing on Antipsychotic Drugs and Cognitive Function in Schizophrenia
DOI:
https://doi.org/10.33192/smj.v77i12.277103Keywords:
In silico study, Functional network analysis, Antipsychotic drugs, Cognitive function, SchizophreniaAbstract
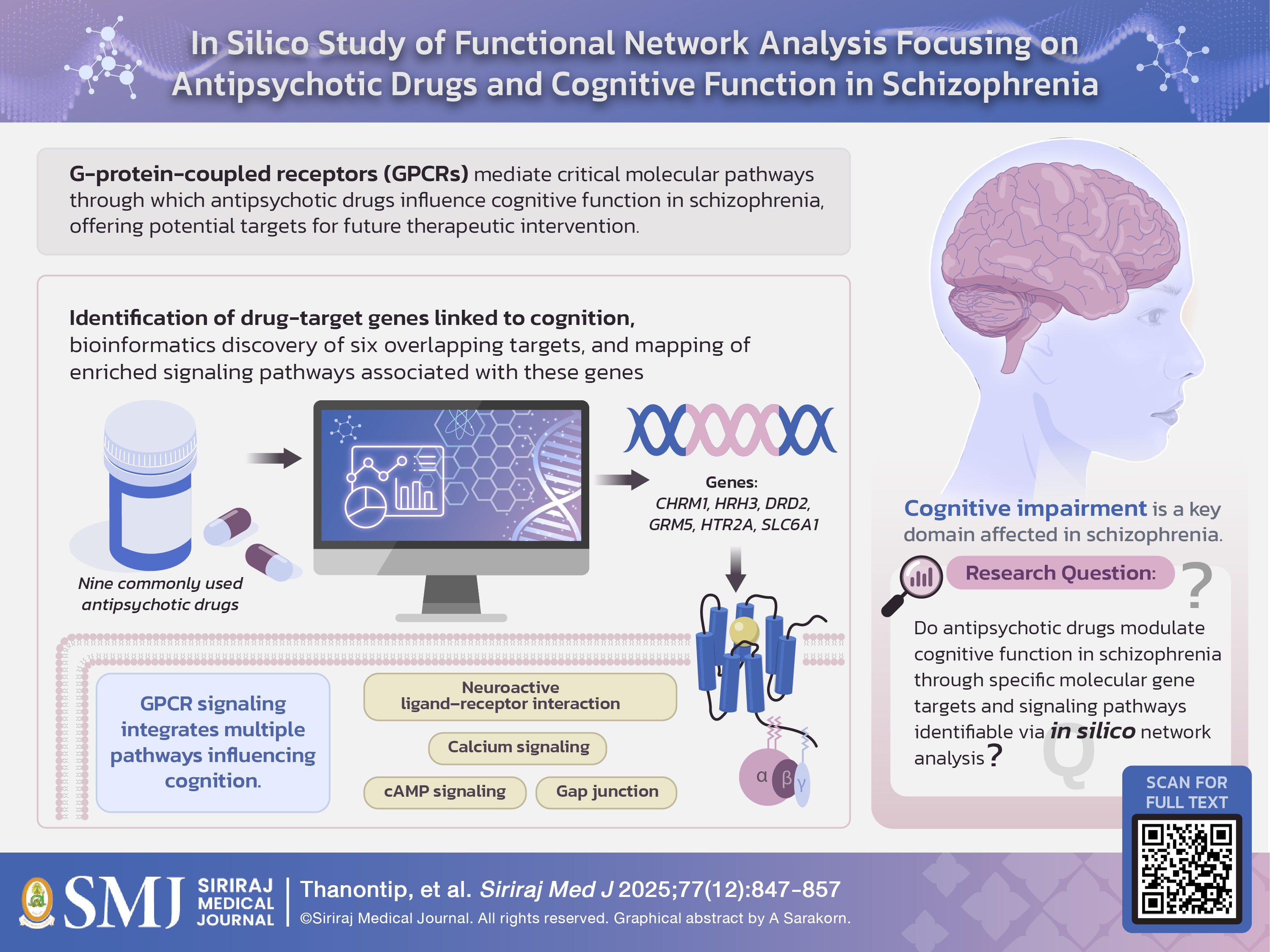
Objective: Recent evidence has emphasized cognitive dysfunction in psychiatric disorders. This study explores bioinformatics analysis aimed at investigating gene targets and pathways related to antipsychotic drug effects on cognitive function in schizophrenia. The main purpose of conventional antipsychotics is to treat the positive symptoms, but they have limited evidence for the involvement of these drugs in cognitive function. Therefore, this study aimed to explore the pathway for the antipsychotic signaling in the cognitive function of schizophrenia.
Materials and Methods: Nine frequently used antipsychotics were included from the literature review. The GeneCards database was utilized to identify the drug target genes associated with cognitive function in schizophrenia. The gene lists were analyzed with two different tools, WebGestalt and STRING. The overlapping genes that result from the two previous steps may play an essential role in cognitive function. These genes were analyzed to find out the related pathway using the KEGG database.
Results: The potential gene list consists of CHRM1, HRH3, DRD2, GRM5, HTR2A, and SLC6A1. The functional enrichment in KEGG reveals six target proteins involved in several pathways, which play an essential role in cognitive function, including the neuroactive ligand-receptor interaction, the gap junction, the calcium signaling pathway, and the cAMP signaling pathway.
Conclusion: The results reveal that G-protein-coupled receptors (GPCRs) are the main target site of potential mechanisms or processes related to antipsychotics and cognitive function. Therefore, GPCRs might be a promising candidate for future research on potential therapeutic targets for intervention in neurological dysfunction-associated cognitive deficits in schizophrenia.
References
Harvey PD, Lombardi J, Leibman M, White L, Parrella M, Powchik P, et al. Cognitive impairment and negative symptoms in geriatric chronic schizophrenic patients: a follow-up study. Schizophr Res. 1996;22(3):223-31.
Hughes C, Kumari V, Soni W, Das M, Binneman B, Drozd S, et al. Longitudinal study of symptoms and cognitive function in chronic schizophrenia. Schizophr Res. 2003;59(2-3):137-46.
Wu JQ, Chen DC, Tan YL, Xiu MH, De Yang F, Soares JC, et al. Cognitive impairments in first-episode drug-naive and chronic medicated schizophrenia: MATRICS consensus cognitive battery in a Chinese Han population. Psychiatry Research. 2016;238:196-202.
Seidman LJ, Buka SL, Goldstein JM, Tsuang MT. Intellectual decline in schizophrenia: evidence from a prospective birth cohort 28-year follow-up study. J Clin Exp Neuropsychol. 2006;28(2):225-42.
Friedman JI, Harvey PD, Coleman T, Moriarty PJ, Bowie C, Parrella M, et al. Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: a comparison with Alzheimer's disease and normal aging. Am J Psychiatry. 2001;158(9):1441-8.
Goldberg TE, Ragland JD, Torrey EF, Gold JM, Bigelow LB, Weinberger DR. Neuropsychological assessment of monozygotic twins discordant for schizophrenia. Arch Gen Psychiatry. 1990;47(11):1066-72.
Riley EM, McGovern D, Mockler D, Doku VC, S OC, Fannon DG, et al. Neuropsychological functioning in first-episode psychosis--evidence of specific deficits. Schizophr Res. 2000;43(1):47-55.
Bilder RM, Lipschutz-Broch L, Reiter G, Geisler SH, Mayerhoff DI, Lieberman JA. Intellectual deficits in first-episode schizophrenia: evidence for progressive deterioration. Schizophr Bull. 1992;18(3):437-48.
Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153(3):321-30.
Sharma T. Quetiapine-efficacy in different domains. Eur Neuropsychopharmacol. 2001;11 Suppl 4:S385-90.
Harvey PD, Lombardi J, Kincaid MM, Parrella M, White L, Powchik P, et al. Cognitive functioning in chronically hospitalized schizophrenic patients: age-related changes and age disorientation as a predictor of impairment. Schizophr Res. 1995;17(1):15-24.
Sharma T, Antonova L. Cognitive function in schizophrenia: Deficits, functional consequences, and future treatment. Psychiatr Clin North Am. 2003;26(1):25-40.
Faber G, Smid HG, Van Gool AR, Wiersma D, Van Den Bosch RJ. The effects of guided discontinuation of antipsychotics on neurocognition in first onset psychosis. Eur Psychiatry. 2012;27(4):275-80.
Knowles EE, David AS, Reichenberg A. Processing speed deficits in schizophrenia: reexamining the evidence. Am J Psychiatry. 2010;167(7):828-35.
Elie D, Poirier M, Chianetta J, Durand M, Gregoire C, Grignon S. Cognitive effects of antipsychotic dosage and polypharmacy: a study with the BACS in patients with schizophrenia and schizoaffective disorder. J Psychopharmacol. 2010;24(7):1037-44.
Green MF, Marder SR, Glynn SM, McGurk SR, Wirshing WC, Wirshing DA, et al. The neurocognitive effects of low-dose haloperidol: a two-year comparison with risperidone. Biol Psychiatry. 2002;51(12):972-8.
Janicak PG, Marder SR, Tandon R, Goldman M. Schizophrenia recent advances in diagnosis and treatment. New York, NY: Springer; 2014.
John MECfCD, Communications S. AHRQ Comparative effectiveness reviews antipsychotic medicines for treating schizophrenia and bipolar disorder: A review of the research for adults and caregivers. Comparative Effectiveness Review Summary Guides for Consumers. Rockville (MD): Agency for Healthcare Research and Quality (US); 2005.
Chokhawala K, Stevens L. Antipsychotic Medications. StatPearls. Treasure Island (FL): StatPearls Publishing LLC.; 2019.
Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho B-C. Antipsychotic Dose Equivalents and Dose-Years: A Standardized Method for Comparing Exposure to Different Drugs. Biol Psychiatry. 2010;67(3):255-62.
GeneCards - Human Genes | Gene Database | Gene Search [cited 2020 8 Feb]. Available from: https://www.genecards.org/.
Webgestalt(Web-based gene set analysis toolkit) [cited 2020 Feb 08]. Available from: http://www.webgestalt.org/.
STRING: functional protein association networks [cited 2020 Feb 8]. Available from: https://string-db.org/cgi/input.pl.
Jenraumjit R, Tethanyawarakool V, Kongkhamboot H, Piyatrakul N. Prescribing Pattern of Antipsychotic Long Acting Injections for the Treatment of Schizophrenia Patients in Suan Prung Psychiatric Hospital. J Psychiatr Assoc Thailand. 2018;63(4):335-48.
Sharma T. Cognitive effects of conventional and atypical antipsychotics in schizophrenia. Br J Psychiatry Suppl. 1999;174(38):44-51.
Sakurai H, Bies RR, Stroup ST, Keefe RSE, Rajji TK, Suzuki T, et al. Dopamine D2 receptor occupancy and cognition in schizophrenia: analysis of the CATIE data. Schizophr Bull. 2013;39(3):564-74.
Knafo S, Venero C. Cognitive Enhancement: Pharmacologic, Environmental and Genetic Factors: Academic Press; 2014.
Dibyajyoti S, Bin ET, Swati P. Bioinformatics: The effects on the cost of drug discovery. Galle Medical Journal. 2013;18(1):44-50.
Buchan NS, Rajpal DK, Webster Y, Alatorre C, Gudivada RC, Zheng C, et al. The role of translational bioinformatics in drug discovery. Drug Discov Today. 2011;16(9-10):426-34.
Wu J, Vallenius T, Ovaska K, Westermarck J, Mäkelä TP, Hautaniemi S. Integrated network analysis platform for protein-protein interactions. Nat Methods. 2009;6(1):75-7.
Bolton JL, Marioni RE, Deary IJ, Harris SE, Stewart MC, Murray GD, et al. Association between polymorphisms of the dopamine receptor D2 and catechol-o-methyl transferase genes and cognitive function. Behav Genet. 2010;40(5):630-8.
Wess J. Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol. 2004;44:423-50.
Barco A, Bailey CH, Kandel ER. Common molecular mechanisms in explicit and implicit memory. J Neurochem. 2006;97(6):1520-33.
Ménard C, Quirion R. Group 1 metabotropic glutamate receptor function and its regulation of learning and memory in the aging brain. Front Pharmacol. 2012;3:182.
Sadek B, Saad A, Sadeq A, Jalal F, Stark H. Histamine H3 receptor as a potential target for cognitive symptoms in neuropsychiatric diseases. Behav Brain Res. 2016;312:415-30.
Zhang G, Stackman Jr RW. The role of serotonin 5-HT2A receptors in memory and cognition. Front Pharmacol. 2015;6:225.
Mitterauer BJ. Possible role of glia in cognitive impairment in schizophrenia. CNS Neurosci Ther. 2011;17(5):333-44.
Kawamoto EM, Vivar C, Camandola S. Physiology and pathology of calcium signaling in the brain. Front Pharmacol. 2012;3:61.
Suwanjang W, Phansuwan-Pujito P, Govitrapong P, Chetsawang B. Calpastatin reduces calpain and caspase activation in methamphetamine-induced toxicity in human neuroblastoma SH-SY5Y cultured cells. Neurosci Lett. 2012;526(1):49-53.
Joshi AU, Kornfeld OS, Mochly-Rosen D. The entangled ER-mitochondrial axis as a potential therapeutic strategy in neurodegeneration: a tangled duo unchained. Cell Calcium. 2016;60(3):218-34.
Titus D, Furones C, Kang Y, Atkins CM. Age-dependent alterations in cAMP signaling contribute to synaptic plasticity deficits following traumatic brain injury. Neuroscience. 2013;231:182-94.
Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, et al. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. 2016;54:1.30.1-1.30.33.
Liao D-L, Hong C-J, Chen H-M, Chen Y-E, Lee S-M, Chang C-Y, et al. Association of muscarinic m1 receptor genetic polymorphisms with psychiatric symptoms and cognitive function in schizophrenic patients. Neuropsychobiology. 2003;48(2):72-6.
Dean B, Soulby A, Evin GM, Scarr E. Levels of [3H] pirenzepine binding in Brodmann's area 6 from subjects with schizophrenia is not associated with changes in the transcription factor SP1 or BACE1. Schizophr Res. 2008;106(2-3):229-36.
Scarr E, Sundram S, Deljo A, Cowie TF, Gibbons AS, Juzva S, et al. Muscarinic M1 receptor sequence: preliminary studies on its effects on cognition and expression. Schizophr Res. 2012;138(1):94-8.
Zhang M, Ballard ME, Pan L, Roberts S, Faghih R, Cowart M, et al. Lack of cataleptogenic potentiation with non-imidazole H3 receptor antagonists reveals potential drug–drug interactions between imidazole-based H3 receptor antagonists and antipsychotic drugs. Brain Res. 2005;1045(1-2):142-9.
Jin C-Y, Anichtchik O, Panula P. Altered histamine H3 receptor radioligand binding in post‐mortem brain samples from subjects with psychiatric diseases. Br J Pharmacol. 2009;157(1):118-29.
Cohen O, Weickert T, Hess J, Paish L, McCoy S, Rothmond D, et al. A splicing-regulatory polymorphism in DRD2 disrupts ZRANB2 binding, impairs cognitive functioning and increases risk for schizophrenia in six Han Chinese samples. Mol Psychiatry. 2016;21(7):975-82.
Nkam I, Ramoz N, Breton F, Mallet J, Gorwood P, Dubertret C. Impact of DRD2/ANKK1 and COMT polymorphisms on attention and cognitive functions in schizophrenia. PloS One. 2017;12(1):e0170147.
Matosin N, Newell KA, Quidé Y, Andrews JL, Teroganova N, Green MJ, et al. Effects of common GRM5 genetic variants on cognition, hippocampal volume and mGluR5 protein levels in schizophrenia. Brain Imaging Behav. 2018;12(2):509-17.
Moghaddam B. Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology (Berl). 2004;174(1):39-44.
Shimizu S, Mizuguchi Y, Ohno Y. Improving the treatment of schizophrenia: role of 5-HT receptors in modulating cognitive and extrapyramidal motor functions. CNS Neurol Disord Drug Targets. 2013;12(6):861-9.
Blasi G, De Virgilio C, Papazacharias A, Taurisano P, Gelao B, Fazio L, et al. Converging evidence for the association of functional genetic variation in the serotonin receptor 2a gene with prefrontal function and olanzapine treatment. JAMA Psychiatry. 2013;70(9):921-30.
Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of γ-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63(10):1372-6.
Janušonis S. Functional associations among G protein-coupled neurotransmitter receptors in the human brain. BMC Neurosci. 2014;15:16.
Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell. 2008;135(3):561-71.
Sassone-Corsi P. The cyclic AMP pathway. Cold Spring Harb Perspect Biol. 2012;4(12):a011148.
Yan K, Gao LN, Cui YL, Zhang Y, Zhou X. The cyclic AMP signaling pathway: Exploring targets for successful drug discovery. Mol Med Rep. 2016;13(5):3715-23.
Pidoux G, Taskén K. Anchored PKA as a gatekeeper for gap junctions. Commun Integr Biol. 2015;8(4):e1057361.
Miyamoto A, Mikoshiba K. Probes for manipulating and monitoring IP3. Cell Calcium. 2017;64:57-64.
Feber L, Peter NL, Chiocchia V, Schneider-Thoma J, Siafis S, Bighelli I, et al. Antipsychotic Drugs and Cognitive Function: A Systematic Review and Network Meta-Analysis. JAMA Psychiatry. 2025;82(1):47-56.
Lee M, Cernvall M, Borg J, Plaven-Sigray P, Larsson C, Erhardt S, et al. Cognitive Function and Variability in Antipsychotic Drug–Naive First-Episode Psychosis. JAMA Psychiatry. 2024;81(5):468-76.
Kelebie M, Kibralew G, Tadesse G, Rtbey G, Aderaw M, Endeshaw W, et al. Effectiveness of antipsychotic medication in patients with schizophrenia in a real world retrospective observational study in Ethiopia. Sci Rep. 2025 Feb 7.
Nnadi CU, Malhotra AK. Individualizing Antipsychotic Drug Therapy in Schizophrenia: The Promise of Pharmacogenetics. Curr Psychiatry Rep. 2005;7(1):1-7.
Begni V, Marchesin A, Riva MA. IUPHAR review-Novel therapeutic targets for schizophrenia treatment: A Translational perspective. Pharmacol Res. 2025;214:107690.
Titus DJ, Furones C, Kang Y, Atkins CM. Age-dependent alterations in cAMP signaling contribute to synaptic plasticity deficits following traumatic brain injury. Neuroscience. 2013;231:182-94.
Faustmann TJ, Corvace F, Faustmann PM, Ismail FS. Influence of antipsychotic drugs on microglia-mediated inflammatory responses in schizophrenia. Front Psychiatry. 2025;16:1522128.
Pitanupong J, Karakate A, Tepsuan L, Sritrangnant G. Attitudes toward long-acting injectable antipsychotics among schizophrenia patients in southern Thailand: A multihospital-based cross-sectional survey. Siriraj Med J. 2022;74(3):193-201.
Ueapanjasin P, Thavornwattanayong W, Lertsirimunkong J, Chaiyakittisopon K.Cost-effectiveness analysis of long-acting injectable once-monthly of aripiprazole compared with long-acting injectable once-monthly paliperidone palmitate for the treatment of stable schizophrenia patients in Thailand. Siriraj Med J. 2023;75(10):725-35.
Downloads
Published
How to Cite
Issue
Section
Categories
License
Copyright (c) 2025 Siriraj Medical Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following conditions:
Copyright Transfer
In submitting a manuscript, the authors acknowledge that the work will become the copyrighted property of Siriraj Medical Journal upon publication.
License
Articles are licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND 4.0). This license allows for the sharing of the work for non-commercial purposes with proper attribution to the authors and the journal. However, it does not permit modifications or the creation of derivative works.
Sharing and Access
Authors are encouraged to share their article on their personal or institutional websites and through other non-commercial platforms. Doing so can increase readership and citations.














